Invasive Lobular Cancer (ILC)
TBC Invasive/Infiltrating Lobular Carcinoma (ILC)
Invasive / Infiltrating Lobular Carcinoma (ILC)
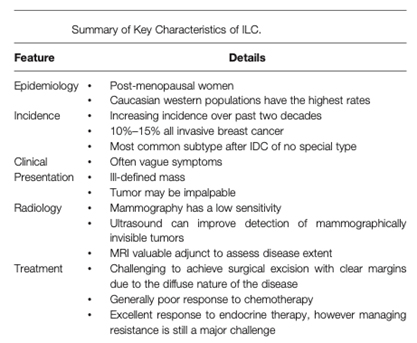
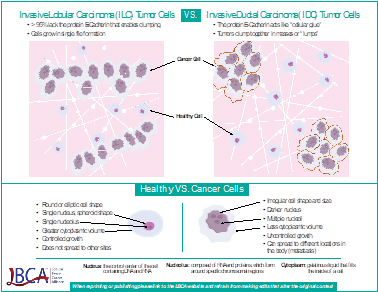
Although invasive lobular cancer is not as common as its ductal counterpart, it warrants specific attention, and a whole section of its own, because of the distinct biological characteristics that affect symptoms, diagnosis, and therapeutic strategies. ILC as a breast cancer subtype continues to pose a challenge in terms of accurate clinical diagnosis, due to its unique histopathology and clinical biology. A hallmark feature of classical invasive lobular breast cancers is that the tumours grow in single-file strands rather than the more common “lump” seen in invasive ductal breast cancers. These characteristic tumour growth patterns of lobular breast cancers are caused by loss of E-cadherin function, a protein that is essential for the cell-to-cell adhesion that promotes normal tissue structure.
The diffuse growth pattern of lobular carcinoma can make diagnosis particularly challenging. Like other breast cancers, lobular carcinomas may be detected before a person feels any symptoms, through regular mammographic breast cancer screenings. Because it tends to form a thickening instead of a lump, lobular cancer can be harder to feel during a breast examination, and clinical signs may be subtle. The characteristic insidious infiltrative process makes ILC difficult to identify radiographically, and the extent of disease challenging to define. Specifically, ILC has a tendency to be underestimated by conventional breast imaging, and a higher likelihood to be multifocal/multicentric, which complicates accurate screening, detection, and treatment. In addition, ILC has historically been thought to confer an increased contralateral breast cancer (CBC) risk. Mammography and ultrasound are less reliable for the early detection of lobular breast cancer, which can lead to later detection, and a more advanced stage at diagnosis, although MRI may be better able to detect lobular carcinoma than mammography.
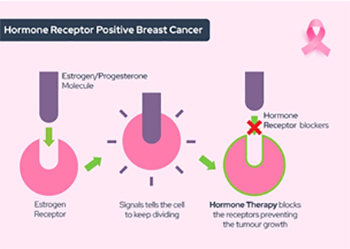
Ninety-five percent of invasive lobular carcinomas are oestrogen receptor (ER) positive and 70 percent are progesterone receptor (PR) positive. Sixty to 70 percent are both ER and PR positive. The majority of ILCs are classified as luminal A-like. The long-term prognosis appears the same for ILC and NST, although there is a tendency towards a higher incidence of late recurrences in ILC. Lobular carcinoma typically expresses low or no HER2 protein (referred to as HER2-negative), so endocrine (anti-hormone) therapies are typically administered for lobular breast cancer treatment. Most commonly, the anti-estrogen therapy tamoxifen is recommended for premenopausal women while an aromatase inhibitor such as letrozole or anastrozole is given to postmenopausal women
Invasive lobular breast cancer (ILC) is the second most common type of breast cancer, behind invasive ductal carcinoma (IDC) of no special type (NST), accounting for around 10-15% of breast cancers. Over the past two decades, incidence rates of lobular carcinoma have increased, mainly among the post-menopausal population, and is likely to be the result of improved diagnostic techniques and the use of hormone replacement therapy. ILC can develop in women of any age, but increasing age increases the risk of invasive lobular carcinoma. According to the American Cancer Society, about two-thirds of women diagnosed with invasive lobular carcinoma are age 55 or older.
Pathology
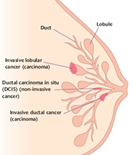
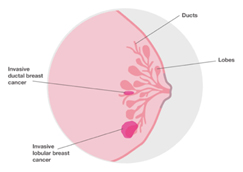
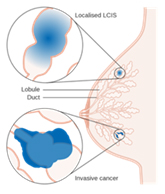
Invasive lobular breast cancer starts in the lobules (milk-producing glands) of the breast, and spreads into the surrounding normal breast tissue. Sometimes invasive lobular breast cancer is found mixed with other types of breast cancer, such as ductal carcinoma in situ (DCIS) or invasive ductal breast cancer.
Remember that if your doctor has told you that you have lobular carcinoma in situ (LCIS), you don’t have invasive lobular breast cancer. They are two different things.
Lobular cancer cells grow as single, separate cells. In the most typical form, called classical, ILC is made up of small cancer cells that invade the connective tissue of the breast and grow in single-file formation. If the cancer cells grow in a different pattern, the ILC may be classified as one of the following histological subtypes:
- Solid: The cells grow in large sheets with little connective tissue between them.
- Alveolar: The cancer cells grow in groups of 20 or more.
- Histiocytoid: the cancer cells look like histiocytes or macrophages
- Signet ring cell: The tumour contains some cells that are filled with mucin and the nucleus is pushed to the side of the cell, giving it a signet ring-like look.
- Pleomorphic: The cancer cells are much larger than the classic ILC cells
The unique growth patterns of lobular breast cancer are caused by a genetic alteration in the CDH1 gene that codes for E-cadherin, a protein that is essential for cell-to-cell adhesion that promotes normal tissue structure. The classic hallmark of ILC, found in 85–95% of cases, is loss of E-cadherin protein expression. E-cadherin is a test that the pathologist might use to help determine if the tumour is ductal or lobular. The cells in invasive lobular carcinomas are usually negative for E-cadherin. If your report does not mention E-cadherin, it means that the test was not needed to tell what type of cancer you have. Instead of clustering together, lobular cancer cells spread out single file like tree branches or spider webs or mesh, and in fact a hallmark feature of classical invasive lobular breast cancers, is that the tumours grow in these single-file strands.
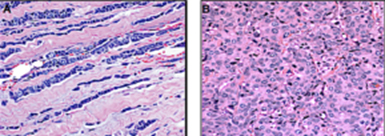
ILC cells (A-above left) form a distinct, single-file pattern that is very different to the more common ductal breast cancer cells (B-above right).
https://lobularbreastcancer.org
Clinical Findings
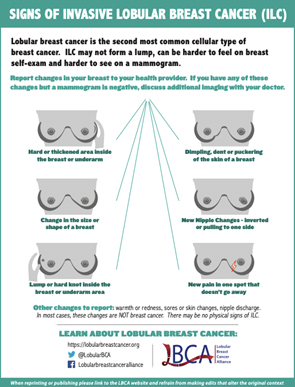
Because the ILC cells don’t stick together well, there’s often no lump, making it harder for women to find during self-exams. Instead, they may notice a thickening or a small “dimple” as the skin is tugged from the inside. Invasive lobular breast cancer may not cause any obvious changes to the breast. You may notice a hardened or thickened area of breast tissue rather than a definite lump.
Possible symptoms include:
- an area of thickening or hardening in the breast (rather than a distinct lump)
- swelling or fullness of all or part of the breast
- a change in the nipple, for example it might become inverted
- a change in the skin, such as dimpling or thickening
- a lump or swelling in the underarm area
https://lobularbreastcancer.org
Imaging
ILC have diffuse growth patterns that just slightly disturb the normal tissue architecture, making them harder to detect by physical examination or mammography. ILC can therefore be more difficult to diagnose than other types of breast cancer, as there may be no obvious symptoms. They may be detected on routine mammography, so in some women, invasive lobular breast cancer is found when attending for breast screening. Mammograms can however give false negative results, as some ILC can be difficult to see on a mammogram, as they look similar to normal tissue. Well-formed masses and calcium deposits aren’t as common as in IDC, making it hard for a radiologist to distinguish ILC from normal breast tissue on a mammogram. Mammograms have been found to have a low sensitivity (57 to 79 %) in detecting ILC, with up to 30 % of cases not visualized at all on mammography, and 35 % of lesions only visible on one view.
Digital mammography (DM) is the standard imaging modality for breast cancer diagnostics. A lower sensitivity of DM is related to, for example, high breast density, low patient age and lobular type of cancer. Over the years, mammography has improved and newer techniques have been developed. Probably the best known of those techniques is Digital Breast Tomosynthesis (DBT), or what is referred to as 3D mammography. While 3D mammography/tomosynthesis improves cancer detection in women with fatty, scattered fibroglandular density and heterogeneously dense breasts, it does not significantly improve cancer detection in women with extremely dense breasts.
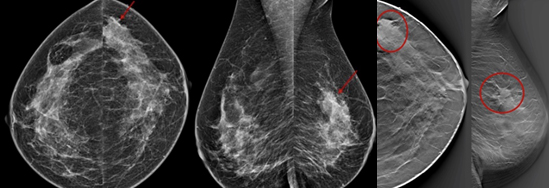
Above left: Standard two view 2D mammogram. The cancer, in the area marked by red arrows, is not well seen.
Above right: Irregular mass (in area of red ovals) is much better seen on the tomosynthesis (3D) slices than the 2D images.
The sensitivity of mammography for the detection of all types of invasive breast carcinomas ranges from 63 to 98 %. Due in part to the histopathologic features of ILC described above, the sensitivity of mammography in detecting ILC is lower, ranging between 57 and 81 %, with false negative rates as high as 25%, so lobular tumours are often discovered at a later stage. If you have dense breasts, this can be a double whammy, as it is well documented that the degree of fibroglandular tissue density is inversely correlated with mammographic sensitivity. When breast tissue is described as heterogeneous or extremely dense, the sensitivity of mammography for the detection of invasive tumours can be as low as 30 to 48 %. A study specifically examining the performance of mammography as a function of both tumour type and breast density, found that mammographic sensitivity was 81 % for IDC, compared with 34 % for ILC. When only those patients with dense breast tissue were considered, sensitivities decreased dramatically to 60 % and 11 %, respectively. Due to these diagnostic challenges, it is crucial for breast imaging radiologists to be aware of the atypical and subtle mammographic patterns of ILC.
The majority of ILC (68 %) present as asymmetric densities or as masses with poorly defined margins, and a well-circumscribed mass is an uncommon mammographic presentation of ILC, seen in less than 1 % of lobular tumours. Overall, the most common mammographic manifestations of ILC include spiculated, ill-defined masses and poorly defined asymmetric densities. Both types of lesions are classically considered suspicious. Why then is the sensitivity of mammography for detecting ILC so low? The answer almost certainly is due to the lack of a conspicuous difference in density from surrounding breast parenchyma.
Apart from spiculated, ill-defined masses and densities, the most common mammographic manifestation of ILC is architectural distortion, which accounts for approximately 14 to 25 % of cases of mammographically detected ILC. 3D mammography or digital breast tomosynthesis (DBT), is better at catching the architectural distortion, as it reduces the tissue-masking effect and improves lesion conspicuousness with a better evaluation of parenchymal distortion, asymmetries and ill-defined masses, which are common findings in ILC. Calcifications, which are readily detected on mammography, are rarely seen in ILC. The frequency of calcifications associated with ILC ranges from 1 to 25 %. Calcifications are easily detected on mammography, due to their high density, which is in noticeable contrast to background breast parenchyma. Even in extremely dense breast tissue, calcifications are typically clearly evident and prompt further investigation. The frequent absence of calcifications in ILC is an additional factor which contributes to the low sensitivity of mammography in detecting these tumours.
The most common appearance of ILC on ultrasound is a hypoechoic mass with posterior acoustic shadowing, occurring in up to 60 % of cases. However, posterior acoustic shadowing may be lacking in up to 20 % of cases. Lobular tumours can also manifest merely as an area of posterior acoustic shadowing without an associated visibly distinct mass. In one series, 15 % of ILC were described as an ‘ill-defined area of altered, hypoechoic, inhomogeneous echotexture without identifiable margins and without frank shadowing’. ILC is rarely seen sonographically as a well-circumscribed mass, reported in only 2 to 12 % of lobular tumours.
Finally, as on mammography, ILC can escape detection on sonographic interrogation, and over 10 % of ILC tumours are sonographically occult. The overall sensitivity of ultrasound for the detection of ILC is reported to be between 68 and 98 %. The higher incidence of nodal disease at presentation in ILC is well documented, however, the optimal imaging method for pre-operative nodal staging in ILC has not been clearly defined. The sensitivity of ultrasound-guided fine-needle aspiration of lymph nodes in ILC is low, reported to be less than 40% in cases of pure ILC.
ILC can sometimes be more difficult than other types of breast cancer not only to locate, but also to accurately measure using mammogram or ultrasound, and may appear smaller than it actually is. MRI is more sensitive than conventional imaging, at detecting ILC, with an overall sensitivity of 93 %, which is similar to the detection of breast cancers overall (90%). MRI can also often provide a more accurate picture of the size, whether it affects more than one area in the breast, and MRI also checks the other breast. Studies have repeatedly shown that MRI is superior to conventional imaging, not only in terms of its increased sensitivity for detecting ILC, but also for the detection of ipsilateral and contralateral disease.
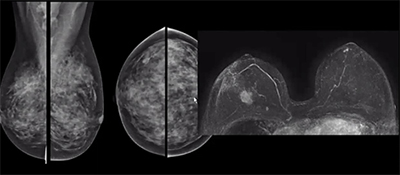
Comparison of a standard mammogram (above left) with dense breast tissue and breast MRI (above right), clearly showing a cancer that is not visible on the mammogram.
In a meta-analysis of studies of MRI use in women with ILC, MRI detected additional ipsilateral disease (disease in the same breast) in 32 % of cases, and 7 % of patients were found to have contralateral disease (disease in the opposite breast). A study assessing the impact of preoperative MRI on the lumpectomy re-excision rate in ILC, found that patients who had an MRI had significantly lower re- excision rates compared with patients without preoperative MRI (9 % versus 27 %, respectively).
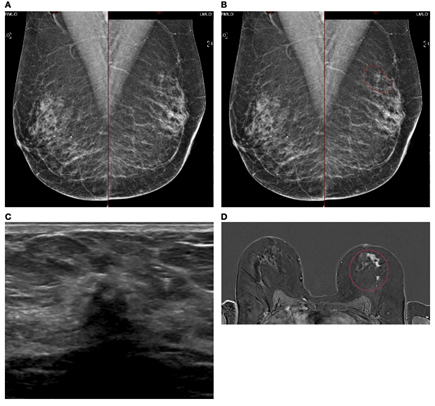
A case of extensive ILC in a 50-year-old female attending for 1st breast screening. (A) Mammogram: Right and left MLO views. (B) Mammogram is reported as M3. There is a 5-mm area of concern on the left which is circled. The calcifications are considered benign. The patient does not feel a palpable mass. (C) An US of the left breast is performed and shows a small 8mm focus close to the nipple. (D) MRI breast follows and this shows an extensive area measuring approximately 50 mm × 50 mm (circled).
Biopsies were performed and showed Gr2 ILC ER8 PgR8 HER negative. Patient had mastectomy and sentinel node biopsy (SNB) that showed 52-mm Gr2 ILC ER8 PgR8 HER2 negative SNB 0/3.
Contrast enhanced breast MRI is known to be the most sensitive test for detecting breast cancer. Contrast is administered, which enables imaging of the new blood vessels that form around cancers—even sometimes before the tumour itself can be seen. MRI detects approximately twice as many cancers as mammography. Unfortunately, MRI is expensive and not readily available to all women. Contrast Enhanced Mammography (CEM), which uses the same principle of enhancing tumour vessels to find those cancers that are hard to detect, is an emerging iodine-based modified mammography technique, which combines conventional mammography with the intravenous administration of an iodinated contrast material, offering both morphological and functional information of breast tissue. This technology is used on a digital mammography machine that that allows the contrast-enhancing lesions to be seen, while also providing a regular mammogram. The CEM exam is significantly less expensive than MRI, and takes only a few minutes longer than a regular mammogram once the dye is given. Iodinated contrast agents however carry some risks. Women with poor kidney function should avoid it and allergic reactions can occur.
In addition to having the same advantages as standard full-field digital mammography (FFDM), CEM provides information regarding tumour enhancement, relying on tumour angiogenesis, similar to dynamic contrast enhanced magnetic resonance imaging, and preliminary studies indicate that CEM is similar to MRI in detecting and staging ILC. Many existing mammography units can be upgraded with CEM equipment. The increase in radiation dose of CEM compared to a FFDM ranges from 106% to 180%. Exam time and reading time are low, and patients with claustrophobia or implantable devices that are not compatible with MRI, can safely perform the exam. CEM evaluation is however limited to the breast, whereas breast MRI also allows the evaluation of the axilla, chest wall and internal mammary chain.
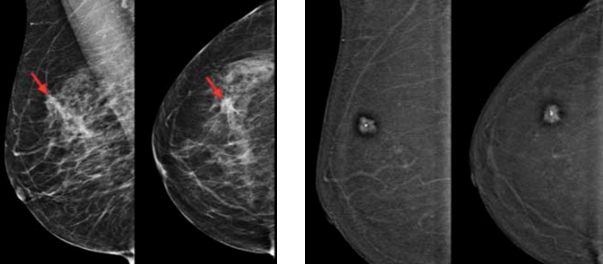
Above left: MLO and CC views of a standard digital mammogram with cancer (red arrows) difficult to detect
Above right: Iodine image in same patient with enhancing cancer clearly visible.
Multifocality / Multicentricity
Lobular carcinoma also tends to be “multifocal” or “multicentric”, that is, patients often have more than one tumour per breast, and if this is the case, your breast surgeon may recommend a mastectomy, but this will depend on the position of the areas affected, and the size of your breast. “Multifocal” means more than area, but only in one quadrant of the breast or the tumour foci are within 5cm of each other, and “multicentric” means there is more than one area of breast cancer in different quadrants of the breast or when the tumour foci are separated by more than 5 cm.
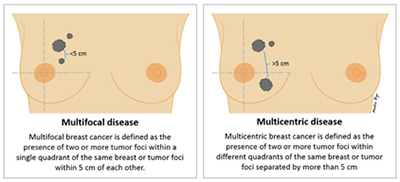
American Joint Committee on Cancer TNM guidelines are used to stage all breast cancers, regardless of histology. The assessment of tumour size (T) category may be more complicated in ILCs, which often present in a multifocal or multicentric fashion. In such cases, the T category is based on the size of the single largest mass, not an additive sum of multiple tumours. Many studies, including a large Surveillance, Epidemiology, and End Results (SEER) registry analysis of 263,408 patients with IDC or ILC, report that patients with ILC are more likely to present with tumours measuring greater than 2cm at the time of diagnosis, as compared with IDC. It is also well documented that the invasive lobular histology is an independent predictor for the likelihood of nodal micrometastases.
Even after an MRI scan, it can sometimes be difficult to estimate the size of an invasive lobular breast cancer before surgery. Because of this, some women who undergo breast- conserving surgery for ILC may require a second operation to achieve adequate pathological margins. In some cases, a mastectomy may be recommended as the second operation. Although the rate of positive margins after lumpectomy for lobular cancer is higher than with ductal carcinoma, the standard of care for treatment of hormone receptor positive ILC is typically no different than for hormone receptor positive IDC.
Molecular Characteristics of ILC / Receptor Status
Most invasive lobular cancers are hormone receptor positive, which means that your doctors are likely to recommend you have hormone therapy. Lobular carcinoma generally does not respond well to chemotherapy because of its biological characteristics.
- Ninety-five percent of invasive lobular carcinoma cancers are oestrogen receptor (ER) positive and 70 percent are progesterone receptor (PR) positive.
- Sixty to 70 percent are both ER and PR positive.
- Most ILC is classified as luminal A in terms of its molecular subtype: ER positive, PR positive, low proliferation index (Ki67) and HER2 negative.
- The lowest rates of ER positivity are observed in pleomorphic ILCs (10%) .
- Most invasive lobular breast cancers are HER2 negative. (8% HER2+, more frequent in pleomorphic lobular cancers).
- HER2 overexpression is rare, seen in only 3 to 5% of classical ILCs, but present in up to 80% of the more aggressive pleomorphic subgroup.
- In ILC, the basal subtype (triple negative: ER, PR and HER2 negative) occurs less frequently, and is also linked to the pleomorphic histological subtype
Risk Factors
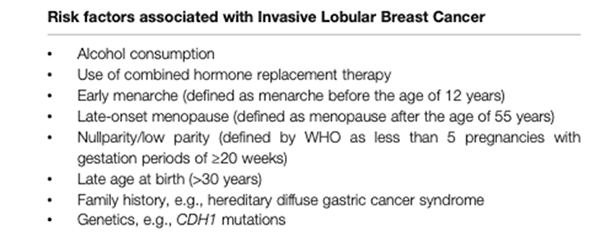
Lifetime exposure to oestrogen is a risk factor for breast cancers that rely on oestrogen for growth. Factors that increase exposure to oestrogen and progesterone, therefore, can increase risk of lobular breast cancer. These factors may include the use of combined hormone replacement therapy and ages at menarche (first menstrual period), childbirth, and menopause—all of which contribute to lifetime hormone exposure. This relationship is also observed for most IDCs, but is more pronounced for ILC. Alcohol consumption, which is a known risk factor for all breast cancers, appears to have a greater impact on risk of invasive lobular carcinoma compared with invasive ductal carcinoma.
Hereditary breast cancer is rare in patients with ILC (<5%), but may be seen in families with hereditary diffuse gastric cancer syndrome, caused by a germline mutation in the tumour suppressor gene, CDH1. ILC otherwise accounts for a minority of cancers associated with known susceptibility genes, comprising less than 10% of cancers in patients with BRCA2 mutations, and less than 5% of cancers in patients with BRCA1 or TP53 mutations.

Associated lobular neoplasia (LN), which refers to the noninvasive proliferative lesions inclusive of atypical lobular hyperplasia (ALH) and lobular carcinoma in situ (LCIS), is observed in more than 50% of classical ILCs. The reported incidence of pure LN ranges from 0.5% to 4%, and typically presents in younger women than does ILC. LN is considered a risk factor for the subsequent development of invasive cancer of either the ductal or the lobular phenotype. The increased risk ranges from 1% to 2% per year, and is conferred equally to both breasts. Recent work demonstrating shared molecular alterations between LCIS and synchronous ILCs in a significant proportion of cases has also reopened the notion that some LCIS lesions may behave as nonobligate precursors of ILC.

Having breast cancer in one breast means the risk of developing a new primary breast cancer in the other breast is slightly higher than in someone who has never had breast cancer. With invasive lobular breast cancer, this risk is widely held to be slightly higher than with other types of breast cancer, but is still low overall. Some series report a higher incidence of bilateral breast cancer, although this finding has not been consistently demonstrated across all studies. Historically, ILC has been associated with higher rates of contralateral breast cancer (CBC) than IDC, with reported risk ranging from 1.8 to 4.3 times higher, however, most of the studies are dated and frequently included lobular carcinoma in situ. Recently, a large cohort database review found no difference in the risks of CBC in ductal and lobular histologies.
If your invasive lobular breast cancer wasn’t originally seen on a mammogram, you may be concerned that follow-up mammograms won’t be effective in detecting changes in your breast, however mammograms are still useful in picking up changes, and just because imaging may not be as good, it doesn’t mean it’s completely useless. In addition, consideration may also on occasions be given to MRI surveillance.

Current Treatment of Invasive Lobular Breast Cancer
Current treatment for ILC follows similar treatment protocols as for other histological subtypes of breast cancer.
Both primary tumours and axillary lymph node (ALN) metastases tend to be non-palpable and difficult to detect by imaging, or fine-needle aspiration or core needle biopsy. The sensitivity of axillary ultrasound imaging for detection of ALN metastases is lower in ILC, especially in patients with a high metastatic nodal burden. Patients with ILC are often diagnosed at a more advanced stage, have a tendency towards having four or more ALN metastases, and more non-sentinel lymph node (SLN) metastases. Despite these differences, current treatment guidelines are similar for ILC and NST.
Surgery
In most cases of breast cancer, breast-conserving surgery by wide local excision is performed to remove the tumour with a margin of the surrounding normal tissue. However, in ILC, there is evidence that 17–65% of patients have to undergo a second surgical intervention. This raises concerns about the accuracy of ILC assessment due to its diffuse and multifocal morphology. Consequently, patients with ILC are more likely to undergo breast removal by mastectomy. Patients with ILC more frequently choose to undergo contralateral mastectomy because of the well-publicised and generally accepted tendency for ILC to be more commonly bilateral than IDC and also because of concerns that a contralateral malignancy if it occurred, would not be easily identified on surveillance breast imaging.
The innately infiltrative growth pattern of ILC and difficult preoperative assessment of extent of disease have historically led surgeons to question the feasibility of breast conserving therapy (BCT) in ILC. When negative margins are achieved, patients with ILC are no more likely to experience locoregional recurrence (LRR) after BCT, with contemporary rates of 3.1% to 5.7%. As defined by the recent consensus guidelines, “negative” margins are defined as no ink on tumour and include a subset analysis showing no benefit to a wider margin for ILC. Based on these findings, the consensus panel concluded that these general recommendations should not be altered for lobular histology.
Neoadjuvant Systemic Therapy
Neoadjuvant chemotherapy (NAC) is aimed at improving rates of breast-conserving surgery. However, consensus has emerged that ILC tends to respond poorly to chemotherapy, leading to lower disease-free and overall survival following neoadjuvant chemotherapy compared with IDC. Histological evidence indicates that ILC persists in up to 99% of cases (i.e., only 1% rate of pathological complete response) following anthracycline-based neoadjuvant chemotherapy. The poor response to chemotherapy is also associated with its luminal A subtype (ER/PR positive, low mitotic index/Ki67). Neoadjuvant chemotherapy is generally not considered for patients with early operable, ILC that is not locally advanced, as it is unlikely to improve rates of breast-conserving surgery but exposes patients to toxicities that can be life threatening. As most ILC is luminal A type and hormone responsive, treatment with endocrine therapy in the neoadjuvant setting may be preferable to chemotherapy.
Patients with locally advanced ILCs should generally receive neoadjuvant therapy before proceeding to surgery. This approach affords an opportunity for downstaging of locally advanced disease without compromising survival. Patients with ILC are significantly less likely than those with IDC to experience a pathologic complete response (pCR) to neoadjuvant chemotherapy (NAC), ranging from 0% to 11%. This is consistent with growing evidence that tumour biology is the principal determinant of response to NAC. The high ER-positivity and low proliferative rates in ILC predispose to a lesser response, a trend seen in most ER-positive breast cancers. Recent data suggest that although overall rates of pCR are low in ILC after NAC, lack of progesterone receptor expression and poor differentiation may identify those with the highest likelihood of benefit.
Adjuvant Therapy
Adjuvant Systemic Therapy
Adjuvant systemic adjuvant therapy is driven largely by tumour biology, rather than histology. There are no randomized trials examining adjuvant chemotherapy regimens specifically in ILC. Although retrospective analyses do not show any definite reasons to deny adjuvant chemotherapy to ILC patients who otherwise meet indications for treatment, the limited response of classic ILCs to chemotherapy in the neoadjuvant setting suggests low chemosensitivity, and invasive lobular carcinoma (ILC) is traditionally considered less responsive to chemotherapy. A study published in Cancer in 2017 found that adjuvant chemotherapy was not associated with improved overall survival for patients with ER-positive/HER2-negative, stage I/II ILC. According to the St Gallen International Consensus Guidelines, in general, women with stage III, ER-positive breast cancer warrant adjuvant chemotherapy. The Panel specifically recommends chemotherapy in women with four or more affected lymph nodes, including those with lobular carcinoma and/or grade 1 or luminal A breast cancers.
The Oncotype Dx 21-gene Recurrence Score (RS) is predictive of chemotherapy benefit for patients with early stage ER-positive/HER2-negative breast cancer, however although the Oncotype recurrence score (RS) has been validated to identify high-risk patients who benefit from chemotherapy, some studies have questioned its relevance in patients with ILC. Studies looking at recurrence scores show a lower percentage of patients with ILC versus those with IDC have a high RS, defined as >25 ( approx. 6.6% vs 16.0%). While studies have shown that a high recurrence score is prognostic for overall survival (OS) in ILC, at least two studies have failed to show that adjuvant chemotherapy is associated with improved OS in high-risk/RS ILC.
Generally, patients with hormone receptor–positive cancers receive endocrine therapy, applicable to the vast majority of ILCs. Chemotherapy is offered for locally advanced cancers and considered for early-stage cancers with high-risk features such as large size, nodal involvement, high grade and more aggressive tumour biology.
Although studies specific to ILC remain limited, some data suggest a greater benefit with aromatase inhibitors compared with tamoxifen, and many patients with ILC have grown concerned that tamoxifen may be less effective for their cancers. This stems from the BIG 1-98 trial analysis, that showed a higher proportional advantage of aromatase inhibitors over tamoxifen for postmenopausal patients with ILC, compared to those with IDC. This was a retrospective analysis performed on a large clinical trial that only included postmenopausal patients. Tamoxifen is still the standard of care for many premenopausal women with early-stage breast cancer. We should not extrapolate from the BIG 1-98 analysis that all women with ILC are somehow resistant to tamoxifen. In general, there is an advantage to aromatase inhibitors over tamoxifen for postmenopausal patients with hormone receptor positive breast cancer; it is just that there was a higher proportional advantage for those with ILC, on the BIG 1-98 study, and further research is required.
Adjuvant Radiotherapy
BCT by definition includes margin negative lumpectomy followed by adjuvant radiotherapy (RT). Adjuvant whole-breast RT reduces the risk of both LRR and death from breast cancer. Similar to surgical trials, ILC patients comprise a minority in RT trials. Existing data support consideration of RT, using existing criteria, regardless of histology.
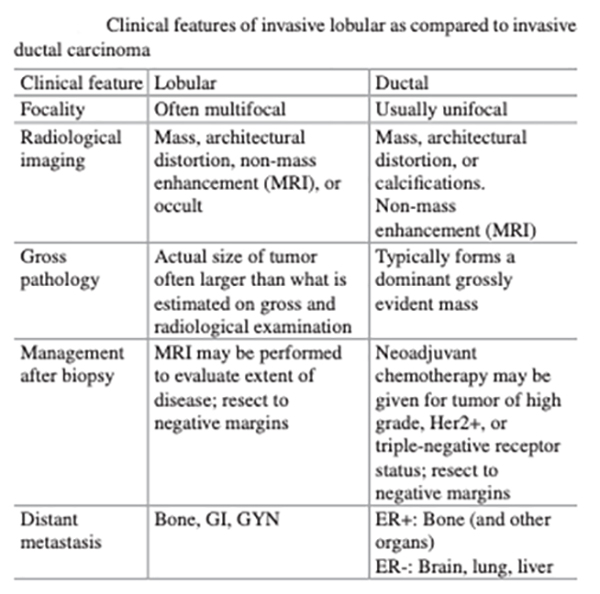
What Does Current Research say about Invasive Lobular Carcinoma of the Breast?
Invasive Lobular Breast Cancer: Data to Support Surgical Decision Making
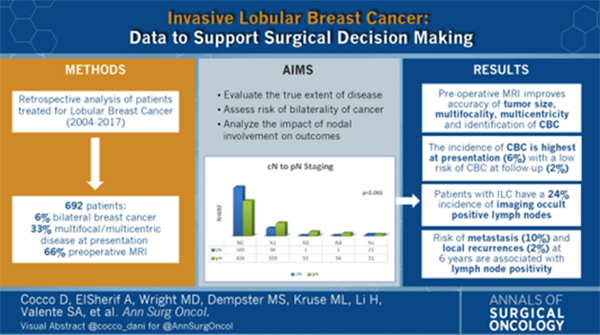
Annals of Surgical Oncology 2021
A study published in 2021 in Annals of Surgical Oncology, retrospectively analysed 692 treated for ILC between 2004 and 2017, and is one of the largest reporting the presentation, treatment, and outcomes for patients with ILC, providing modern data to which the breast surgeon can refer to when counselling patients in the pre-operative decision-making setting.
The results provide additional insight to inform patients regarding the well-known multicentricity of disease, the lower than historically reported risk of CBC, and the limitations of standard imaging in detecting this distinct, and often imaging occult disease. At presentation, 6% had a diagnosis of CBC, 33% had multifocal/multicentric disease and preoperative magnetic resonance imaging (MRI) led to an identification of additional disease in 20% of the patients. Imaging occult nodal disease was identified in 24% of patients.
ILC has historically been thought to confer an increased contralateral breast cancer (CBC) risk, impacting the rate of contralateral prophylactic mastectomies (CPM). In this series, the incidence of CBC was found to be highest at presentation (6%). The subsequent incidence of CBC among the patients not treated with contralateral prophylactic mastectomy (CPM) was 2.3% during a 6-year follow-up period, equating to 0.3% per year. This modern CBC development risk data should be referenced when physicians discuss CPM for ILC with unilateral disease. Interestingly, similar to other studies, the study showed that after a diagnosis of ILC, 65% of the subsequent CBC cases had invasive ductal cancer (IDC) histology, so the use of postoperative screening with MRI may be unnecessary for disease detection.
The higher incidence of nodal disease at presentation in ILC has been well documented, and was also was observed in this study. Overall, 35% of the patients were found to have node-positive cancer. The optimal imaging method for preoperative nodal staging in ILC has however not been clearly defined. Although ultrasound (US) usually is considered the most sensitive diagnostic tool to detect nodal involvement, findings have shown that preoperative US is inferior for detecting axillary node metastasis in ILC, than it is in IDC. It has been hypothesized that the infiltrative pattern of the metastatic spread of ILC within the lymph node allows for continued preservation of the nodal architecture, thereby making sonographic interpretation of the node challenging. As a result, significantly higher false-negative rates and lower sensitivities were observed with preoperative US for ILC than with IDC.
Results show a 24% rate for imaging of occult nodal disease found at the time of surgery, which is higher than the rate of occult nodal disease (14%) found in other studies evaluating both ductal and lobular estimations. Moreover, studies have shown that occult ILC nodal disease has a 38% likelihood of being upstaged to N2 (4-9 nodes +) or N3 (10 or more nodes +) nodal disease. The data similarly showed a higher rate of pathologic upstaging to advanced nodal disease, with 45% of clinically node-positive patients upstaged to N2 or N3. Increasing T size was significantly associated with the incidence of clinical N0 upstaging to N1 (1-3 nodes +) or higher disease stage.
The concept of lymph node upgrading is extremely important, especially in the operative setting, with patients possibly electing for mastectomy to potentially avoid radiation. For example, among the patients undergoing mastectomy in this study, the incidence of N0 disease upstaged to N1 or higher at surgery, was 30%. Although perhaps no additional nodal surgery would be necessary for such patients based on the degree of pathologic nodal involvement, post-mastectomy nodal radiation may be considered. Therefore, for patients with clinically N0 ILC, undergoing mastectomy with immediate breast reconstruction, a 30% possibility of requiring adjuvant radiation for node-positive disease as in this study, should be discussed with both the patient and the reconstructive surgeon.
During the 6-year follow-up period, 9.4% of the patients included in this study experienced metastatic disease. Although factors significantly predicting metastatic recurrence were not identified, 72% of the patients who relapsed distantly had initial lymph node involvement, which appears to be the highest risk. Although larger tumour size and node-positive disease were not predictive factors for recurrence, tumour size was predictive of occult nodal disease.
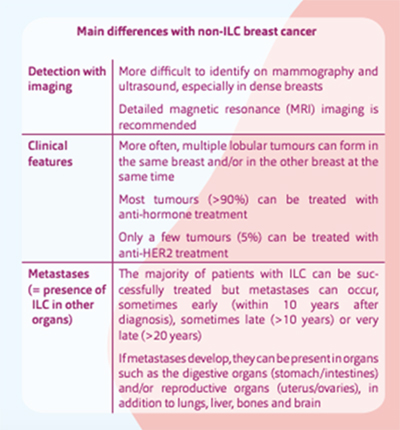
An important difference between ILC and IDC is their pattern of metastatic spread. While both IDC and ILC commonly metastasize to lymph nodes, bone and liver, ILC demonstrates a predilection for metastases to the peritoneum, retroperitoneum, and hollow viscera (including the gastrointestinal and genitourinary tracts). Lung metastases are more common in IDC. The overwhelmingly ER-positive nature of ILCs also results in more frequent development of late metastases.
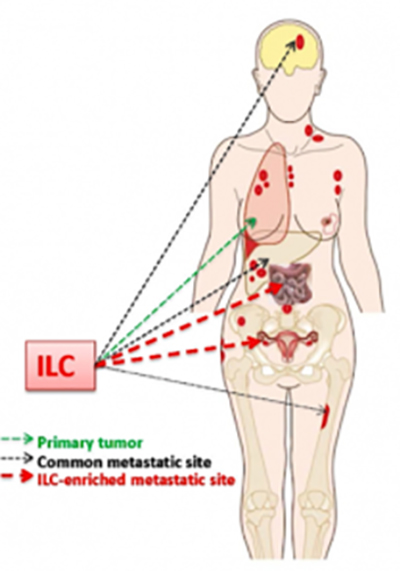
Lobular breast cancer may spread to unusual sites, including the ovaries, abdomen and gastrointestinal tract
Reproduced from abcdiagnosis.co.uk
How is lobular breast cancer imaged if it has spread outside of the breast?
Systemic staging for distant metastases (when cancer spreads to other parts of the body) is done with CT, bone scan or PET/CT. The current standard for systemic staging is CT with bone scan, although data is increasing that detection of distant metastases and evaluation of treatment response in distant metastases may be better performed by FDG PET/CT. Unfortunately however, the impact of PET/CT on systemic staging may be lower for ILC patients than for IDC patients. A study suggests that FDG PET/CT is more likely to reveal unsuspected distant metastases in stage III IDC patients than in stage III ILC patients, and in addition, some ILC patients were upstaged only by the CT component of PET/ CT, because metastases detected on CT were not FDG–avid.
Invasive Lobular Cancer (ILC) Organisations
The Lobular Breast Cancer Alliance (LBCA) is a USA non-profit organization with a network of health care professionals, which works to advance invasive lobular carcinoma (ILC) research, refine treatments and improve patient care.
ELBCA European Lobular Breast Cancer Advocates
Patient Information
References:
2024 Lobular Breast Cancer StatPearls
2023 Lobular Carcinoma of the Breast: A Comprehensive Review with Translational Insights
2023 Efficacy of chemotherapy in patients with HR+/HER2–Invasive lobular breast cancer
2022. Invasive Lobular Carcinoma of the Breast: Toward Tailoring Therapy?
2021 Invasive lobular carcinoma of the breast: the increasing importance of this special subtype
2021 Lobular Breast Cancer: A Review
2020 Invasive Lobular Breast Cancer as a Distinct Disease: Implications for Therapeutic Strategy
2018 Lobular Breast Cancer Different Disease, Different Algorithms?