Your Pathology Report
What is a pathologist?
A pathologist is a doctor who specialises in diagnosing diseases by examining tissue that has been removed from the body. The breast pathologist may be presented with a range of breast specimens, which may be obtained by fine needle aspiration (FNA), core biopsy, or open surgical excision. The initial role of the pathologist is to determine the correct diagnosis. The investigation of a breast abnormality relies on a combination of clinical, imaging and biopsy techniques, which culminate in the examination of a cell or tissue sample by a pathologist.
Samples of your breast tissue removed during needle biopsy or surgery, are sent to a pathologist for examination. A pathologist determines if there is a breast cancer present, and if a diagnosis of invasive breast carcinoma is made, the pathologist is required to provide further information including a range of prognostic and predictive factors that help determine appropriate therapy. A prognostic factor is any measurement available at the time of diagnosis associated with disease free or overall survival such as tumour size or nodal status. A predictive factor is any measurement associated with response or lack of response to a particular therapy, such as an oestrogen receptor positive tumour responding to tamoxifen. For the patient with breast carcinoma, tailoring therapy for the individual has become the aim for all those involved. There is increasing dependence on accurate and reliable pathology reporting to reach this goal. By determining the type of breast cancer and the extent of the disease, the pathologist plays an indispensable role in treating people with breast cancer, and is an integral part of the Breast Cancer Multidisciplinary Team.
If I see a patient who has undergone their preoperative diagnostic core biopsy elsewhere, or I see a patient for a second opinion, who has undergone surgery elsewhere, I have a low threshold to ask our pathologist to review the outside pathology slides, especially if the results reported perhaps don’t quite fit in with the clinical scenario.
All patients are discussed both pre and postoperatively in our multidisciplinary team meetings, and all of the breast imaging, including MRI scans reviewed, so the pathologist is usually familiar with the patient’s case before they even receive the operative specimen, especially obviously if they have reported the preoperative diagnostic core biopsy.
In order to get the best pathological opinion, the clinician should provide the pathologist interpreting the specimen with as much clinical information as possible. It is not some sort of mystery treasure hunt! Detailing the exact nature and site of the specimen is important, and I like all my surgical specimens to be weighed by the pathology lab.
I obtain an intraoperative specimen x-ray on all lumpectomy specimens and any specimens that have undergone preoperative image guided localisation (above right) , and I provide the pathologist with a copy of the specimen x-ray, to enable them to correlate this with the histology. The lumpectomy specimen is marked/oriented with radio opaque clips that show up on the specimen x-ray, so that if it is felt that one of the margins looks to be a little close mammographically on the intraoperative specimen x-ray, further tissue can be taken from that area at the time of surgery.
In addition to the standard pathology request form, I provide the pathologist with a supplementary pathology form containing additional information, including further clinical and imaging details, which I attach to the pathology request form, together with a copy of ALL breast imaging reports including mammogram, ultrasound and MRI, if applicable, as well as the pathology reports on any specimens which may have been done at other labs, such as via Breastscreen.
What is a breast pathology report?
The pathologist prepares a summary report of his findings, which is called the pathology report. The pathology report is provided to communicate the result with the medical practitioner who sent the specimen for pathological evaluation, and as such, is written in highly technical medical language that may be difficult for the patient to fully understand. I routinely provides patients with a copy of their pathology reports, explaining and discussing the report contents. Although the pathology report is about your tissue, its primary purpose is to communicate information to your medical team, in order to enable them to determine the best treatment plan for you, and as such is full of complex medical terminology. Your surgeon will explain the pathology report contents, and their relevance, to you, and you have to be guided by them as to which sections are more or less important than others, as unless you have a scientific background, the pathology report can be pretty difficult to decipher, and you can easily get bogged down in irrelevant detail.
What will you find in a pathology report?
The information
Breast Core Needle Biopsy (CNB) specimen
The first report you are likely to encounter is your breast core needle biopsy (CNB) report. Parts of a breast core needle biopsy (CNB) pathology report and a breast operative pathology report are very similar, with one important difference: the biopsy pathology report will not have information about the size of the cancer, because a biopsy only removes a small piece of tissue. It should be noted, that although the size of the individual core biopsy specimens will often be listed in the CNB pathology report, that measurement does not reflect the actual size of the breast cancer, which cannot be determined until the whole cancer is removed by surgical excision.
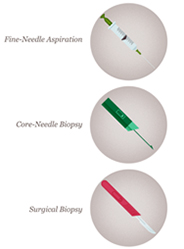
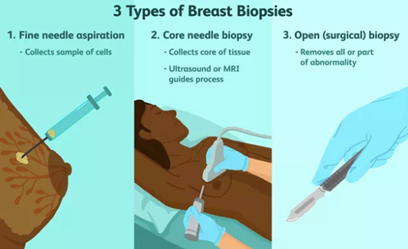
Fine needle aspiration biopsy is a simple, inexpensive, reliable and rapid technique for obtaining cells from a targeted area of breast tissue, whereas a core biopsy involves obtaining single or multiple tissue cores from the breast. Fine needle aspiration (FNA) removes cells from the tumour which are then examined under a microscope, and the cytology report will confirm whether they are malignant (cancerous) or benign (not cancerous). Fine needle aspiration can be used to establish/support a diagnosis of malignancy, but cytological preparations do not provide a reliable distinction between ductal carcinoma in situ (DCIS) and invasive carcinoma and also generally do not allow for the determination of hormone receptor and HER2 status.
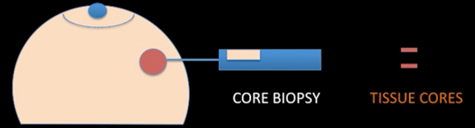
A core needle biopsy (CNB) consists of the removal of tissue specimens with a hollow cutting needle (usually 14 gauge). Obtaining 4 specimens with a 14 gauge needle usually provides enough tissue for diagnosis. A small metallic marker or clip is often placed at the biopsy site so the area can be seen on radiographic imaging studies, assisting the surgeon to localize the tumour.
The advantages of CNB over FNAC include lower sampling error and larger volume of tissue retrieved, allowing the pathologist to document invasive versus in situ/preinvasive disease, estimate the provisional tumour grade more accurately and often perform tumour biomarker tests. The principal aim of a breast core needle biopsy (CNB) is to provide a pathological diagnosis of a breast abnormality before, and in many cases avoiding the need for, open surgical biopsy. How much information is provided by the pathologist from a CNB showing a breast malignancy will vary between pathologists and institutions.

Core biopsy device, needle and sample
Core biopsy removes small cores of tissue, as shown above right, and unlike fine needle aspirate, yields tissue fragments allowing architectural features of the lesion to be identified. Core biopsies are now used in nearly all circumstances to make a breast cancer tissue diagnosis, which allows preoperative assessment of:
- Whether the tumour is invasive or preinvasive ( DCIS)
- Histological subtype eg invasive ductal or lobular carcinoma
- Estimate of the provisional grade- histological grade may be underestimated from the CNB as a result of a lower mitotic rate being seen with the small amount of tissue available for assessment
- Molecular Subtype / Receptor status- ER/PR/HER2- The accuracy of assessment of the predictive factors ER, PR and HER2 in core biopsies when compared with excision specimens is still the subject of debate.
- Lymphovascular invasion (LVI)- may be difficult to detect on CNB, however if identified, it is recommended that this finding is included in the CNB pathology report
All of these results assist your breast surgeon in making decisions about potential treatment, including the sequencing. and whether neoadjuvant chemotherapy should be considered, as well as planning for surgery.
A pathology category classification (B1-5) is used by the UK National Health Service Breast Screening Program (NHSBSP) and by other mammographic screening programs including in Australia. The pathology category given by the pathologist in the report is then used to determine future management of the patient together with the other two components of the ‘triple test’, ie, the clinical examination and history, and the imaging findings.
- B1 (normal tissue): Normal breast or other normal tissue, including adipose tissue
- B2 (benign lesion): FA, fat necrosis,
- B3 (lesion of uncertain malignant potential): Includes ADH, LN, fibroepithelial lesions with cellular stroma and phyllodes tumours (PTs), papillary lesions, FEA and radial scar.
- B4 (suspicious): A definite malignant diagnosis (DCIS or invasive carcinoma) is not possible because of crush artifact, poor fixation or a small questionable focus of non-diagnostic cells.
- B5 (malignant): An unequivocal malignant diagnosis (includes DCIS and invasive carcinoma)
The management of the various B3 lesions has been the subject of a number of studies designed to establish which lesions require surgical excision and which can be followed-up without surgery. Currently, a large majority of these lesions is managed by open surgical excision.
Operative Breast Specimen
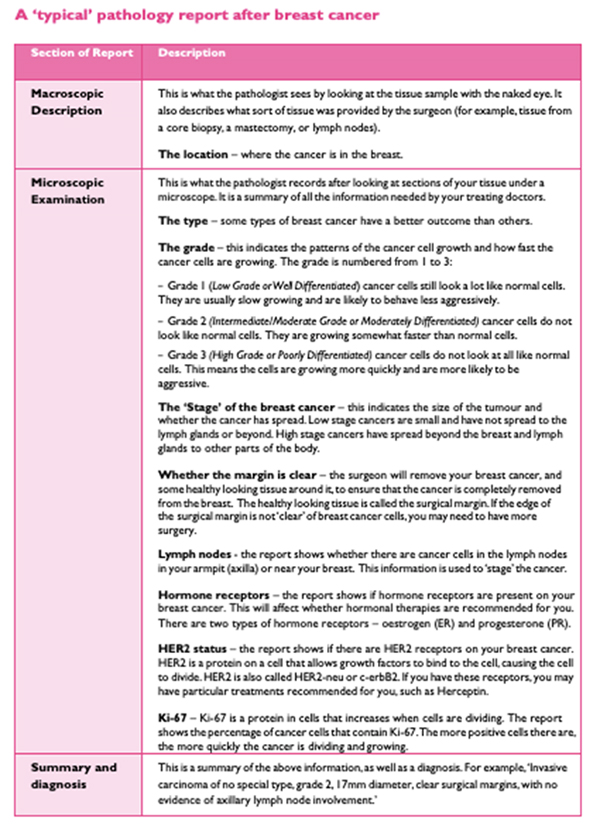
The operative pathology report is broken down into several sections, including some information about the patient, such as the clinical diagnosis (suspected or known), the procedure, a description of what the specimen looks like to the naked eye (called macroscopic description), a description of what was seen under the microscope (microscopic description), and a pathologic diagnosis.
Your pathology report starts with general information such as your name, date of birth and hospital number, as well as your doctor’s name and the date of your surgery. This is usually followed by a description of the breast tissue before it’s looked at under a microscope. This section of the report is called the gross or macroscopic description and includes information about:
- The size, weight and appearance of the tissue
- Site: where it was in the breast before it was taken out
- How it was prepared for the microscope
Next follows the microscopic description, which points out all the features of the cancer seen under a microscope. Finally, there is a summary of the main points, sometimes in a list at the end of the report.
In the case of a breast cancer, the pathologist will describe the type of cell the cancer comes from, the tumour size and grade, whether the cancer cells have entered the lymph channels or blood vessels, information about surgical resection margins, and hormone receptor and HER2 status. Breast cancer pathology reports are one of the more complex pathology reports and can seem quite overwhelming at first glance.
Macroscopic
This is generally not that important to you, the patient. It is a description of what the pathologist received and sees with the naked eye. In a biopsy, the specimen is likely a small, nondescript piece of tissue, in which case the pathologist may describe the colour, shape, feeling and size of the tissue. After a breast cancer surgery, large pieces of tissue and lymph nodes may be submitted and described in the report. This description might report the weight of the specimen, the presence of “inked” margins or sutures, which the surgeon adds so the pathologist can tell “which end is up” once the tissue is disconnected from the body.
There may be mention of surgical clips or wires that were used by the surgeon to be sure that the suspicious area was removed. After a sentinel node biopsy, the gross description may say a lymph node is “hot”, which refers to the radioactive tracer that is used by the surgeon to locate the sentinel node, or that it is “blue”, due to the presence of dye that can also be used to locate the node. The pathologist often then describes how the tissue was divided up for further analysis.
Microscopic
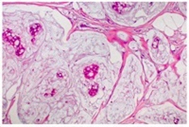
This section contains the most useful information. Not every report goes through the microscopic diagnosis in the same order and some use different terms to describe the same thing. In this section we will discuss each part of the microscopic description in detail. Sometimes the tests are performed in different laboratories or take different lengths of time to complete, which can mean you may not get all the results at once. It is important to wait for all the results to best understand your situation.
Type of Breast Cancer
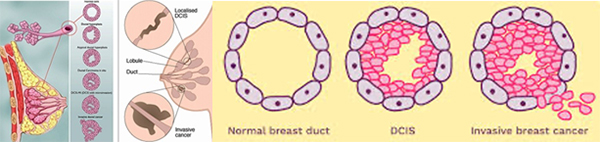
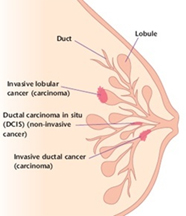
It seems simple: we are talking about breast cancer, so that’s what type it is. But it is not so simple. Almost all breast cancers arise from glandular tissue, making them adenocarcinomas (cancer of the glandular tissue); they are further named by where they start in the breast and how they appear under the microscope. To better understand this section, you need to have some knowledge of normal breast tissue. Breast tissue is composed of lobules, which produce milk; and ducts, which carry the milk to the nipple. Breast cancer starts in a duct or a lobule and this, along with its appearance under the microscope, determine the type of breast cancer it is. The type can dictate some of the treatment choices, although many types are treated similarly. In addition to the type, the cancer can be non-invasive, which means it does not spread beyond the lobule or duct, or invasive, which means it has spread beyond the lobule or duct.
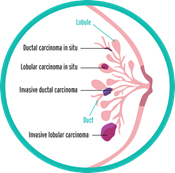
Types of Non-Invasive Breast Cancer
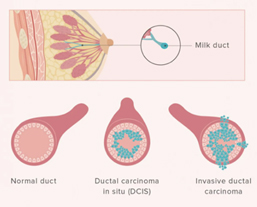
Ductal carcinoma in situ (DCIS)
Ductal Carcinoma In Situ (DCIS)
DCIS is the most common type of non-invasive breast cancer and is sometimes called intraductal carcinoma. It is malignant (cancerous), and as it grows, the centre of the tumour starts to die because it has outgrown its blood supply. This area of dead tissue, called necrosis, can calcify, which can be detected on a mammogram. The pathology report will indicate whether these microcalcifications are present.

DCIS is described as low, intermediate or high grade. In DCIS, grading is based solely on the size of the nuclei of the malignant cells as compared to the nuclear size of normal ductal epithelial cells. Low grade (grade 1) DCIS demonstrates nuclei that are 1.5 to 2 times that of normal ductal cells. The nuclei of intermediate grade (grade 2) DCIS are 2 to 2.5 times the size of normal ductal cells and high grade (grade 3) DCIS demonstrates nuclei greater than 2.5 times the normal nuclear size. The architectural features of the DCIS will also be described. This may include a micropapillary (fern-like) appearance, cribriform (Swiss cheese-like) appearance, or a solid (completing filling the ducts) growth pattern. If areas of DCIS exist where the cells have outgrown their blood supply and have become necrotic, then the DCIS will be described as having comedo necrosis. The pathology report will indicate whether these microcalcifications are present. See: Ductal Carcinoma In Situ

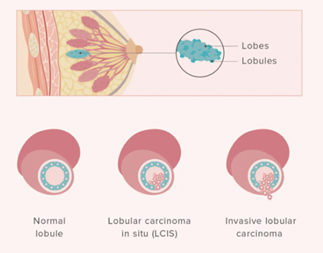
Lobular Carcinoma in Situ (LCIS)
Lobular Carcinoma in Situ (LCIS) 1
Clinical guidance for the management of lobular carcinoma in situ
Lobular carcinoma in situ and atypical hyperplasias of the breast: understanding your diagnosis
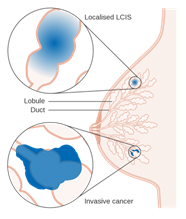
Even though its name includes the term “carcinoma,” LCIS is considered to be a benign breast condition. For this reason, some experts prefer the term “lobular neoplasia” instead of “lobular carcinoma.” LCIS is not considered a true cancer, rather an accumulation of abnormal cells in the lobule. LCIS is considered a risk factor for developing breast cancer in the future in either breast. LCIS is often found incidentally by the pathologist in a tissue specimen that was removed for another reason. LCIS lesions uncommonly develop necrosis or calcifications, so are not often detected on mammograms.

Types of Invasive Breast Cancers
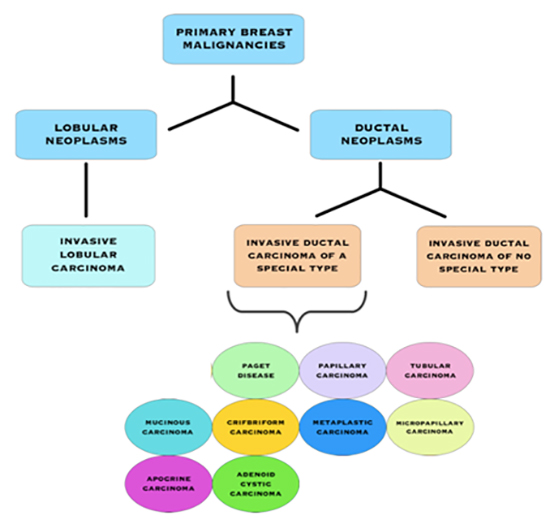
Invasive Breast Cancer Subtypes
Invasive Ductal Carcinoma (IDC) of No Special Type (NST)
Invasive Ductal Carcinoma (IDC) of No Special Type (NST)
IDC is the most common type of invasive cancer, accounting for about 80% of cases. This tumour starts in the duct and spreads beyond the duct into normal breast tissue. Most IDC are “no special type”. You may see this written as NST or NOS (not otherwise specified). Around 70% of breast cancers are this type. It is called ‘no special type’ because the cancer cells have no features that class them as a special type of breast cancer when examined under the microscope. Sometimes invasive breast cancer (NST) is found mixed with other types of breast cancer. Special type breast cancers have cells with particular features.
Some of the special type breast cancers may have a better prognosis than the more common IDC.
These include:
- Adenoid cystic (or adenocystic) carcinoma
- Medullary carcinoma
- Mucinous (or colloid) carcinoma
- Papillary carcinoma
- Tubular carcinoma
Some special sub-types have the same or maybe worse prognoses than IDC.
These include:
- Metaplastic carcinoma
- Micropapillary carcinoma
- Mixed carcinoma (has features of both invasive ductal and invasive lobular)
Invasive/Infiltrating Lobular Carcinoma (ILC)
Invasive/Infiltrating Lobular Carcinoma (ILC)
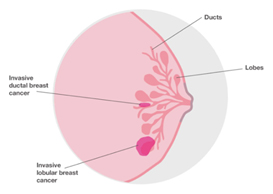
Invasive lobular breast cancer (ILC) is the second most common type of breast cancer, accounting for around 10-15% of breast cancers. Invasive lobular breast cancer starts in the lobules (milk-producing glands) of the breast, and spreads into the surrounding normal breast tissue. Sometimes invasive lobular breast cancer is found mixed with other types of breast cancer, such as ductal carcinoma in situ (DCIS) or invasive ductal breast cancer. See: Invasive Lobular Cancer
E-cadherin is a test that the pathologist might use to help determine if the tumour is ductal or lobular. (The cells in invasive lobular carcinomas are usually negative for E-cadherin.) If your report does not mention E-cadherin, it means that this test was not needed to tell what type of cancer you have.
Remember that if your doctor has told you that you have lobular carcinoma in situ (LCIS), you don’t have invasive lobular breast cancer. They are two different things.
Lobular cancer cells grow as single, separate cells. In the most typical form, called classic, ILC is made up of small cancer cells that invade the connective tissue of the breast and grow in single-file formation. If the cancer cells grow in a different pattern, the ILC may be classified as one of the following subtypes:
- Solid: The cells grow in large sheets with little connective tissue between them.
- Alveolar: The cancer cells grow in groups of 20 or more.
- Histiocytoid: the cancer cells look like histiocytes or macrophages
- Signet ring cell: The tumour contains some cells that are filled with mucin and the nucleus is pushed to the side of the cell, giving it a signet ring-like look.
- Pleomorphic: The cancer cells are much larger than the classic ILC cells
Invasive lobular breast cancer may not cause any obvious changes to the breast. You may notice a hardened or thickened area of breast tissue rather than a definite lump.
Possible symptoms include:
- an area of thickening or swelling
- a change in the nipple, for example it might become inverted
- a change in the skin, such as dimpling or thickening
It can be more difficult to diagnose than other types of breast cancer if there are no obvious symptoms, but may be detected on routine mammography, so in some women, invasive lobular breast cancer is found when attending for breast screening. Some invasive lobular breast cancers can however be difficult to see on a mammogram, as the changes can be very subtle. ILC can sometimes be more difficult than other types of breast cancer to locate and accurately measure using ultrasound or a mammogram, so you may have a magnetic resonance imaging (MRI) scan of your breast. MRI can often provide a more accurate picture of the size of this type of cancer, whether it affects more than one area in the breast, and both breasts are checked.
Invasive lobular breast cancer is sometimes found in more than one area within the breast (multifocal or multicentric). If this is the case, your breast surgeon may recommend a mastectomy, but this will depend on the position of the areas affected, and the size of your breast.
Sometimes, even after an MRI scan, it can sometimes be difficult to estimate the size of an invasive lobular breast cancer before surgery. Because of this, some women who undergo breast- conserving surgery for ILC may need a second operation to achieve adequate pathological margins. In some cases, a mastectomy will be recommended as the second operation.
- Most invasive lobular cancers are hormone receptor positive, which means that your doctors are likely to recommend you have hormone therapy.
- Most invasive lobular breast cancers are HER2 negative.

Having breast cancer in one breast means the risk of developing a new primary breast cancer in the other breast is slightly higher than in someone who has never had breast cancer. With invasive lobular breast cancer, this risk may be slightly higher than with other types of breast cancer, but it is still low overall. If your invasive lobular breast cancer wasn’t originally seen on a mammogram, you may be concerned that follow-up mammograms won’t be effective in detecting changes in your breast, however, mammograms are still useful in picking up early changes, and in addition, consideration may on occasions be given to MRI surveillance also.
Medullary Carcinoma
Medullary carcinoma is rare, accounting for only 5% of all breast cancers. It may also be called invasive breast cancer of no special type with medullary features, or medullary-like cancer. It is called “medullary” because when pathologists first looked at these tumours, they were reminded of the grayish soft tissue in the brainstem, or medulla. They occur more often in younger women and in women who have inherited a faulty BRCA 1 gene. The cancer cells tend to be bigger than in other types of breast cancer, with a clear boundary between the tumour and the normal tissue. Though medullary tumours are usually small, the cells are often high grade. Most medullary breast cancers are triple negative, however, people diagnosed with medullary breast cancer generally have a better prognosis than with some other types of triple negative breast cancer. While each case is different, the outlook for this type of breast cancer is often good.
Tubular Carcinoma
Tubular carcinoma is a rare type of invasive breast cancer, accounting for about 2% of cases. t’s most common in women over 50, although you can get it at any age. Its name comes from the pathologist seeing a “tubular pattern” in 75% or more of the specimen. It’s often found alongside other types of breast cancer. Generally, tubular breast cancer has a very good prognosis following treatment. This is because the cells are nearly always low grade and slow growing. The outlook is particularly good if the cancer is ‘pure’ tubular, and not mixed with other types of breast cancer. Tubular cancer does not typically spread to other areas of the body and is associated with very good prognosis.

Mucinous Carcinoma
Mucinous breast cancer (sometimes called colloid breast cancer) is so called because when it is viewed under a microscope the cancer cells are surrounded by mucus. 1 to 2 % of breast cancers are mucinous breast cancers. This type of cancer tends to be slower growing than other types, and can occur at any age, but is more commonly found in women over 60. It is less likely to spread to the lymph nodes, and is usually low grade. Mucinous cancers are usually positive for the oestrogen and/or progesterone receptors (ER/PR+) and negative for the HER2 receptor (HER2-). It can be a “pure” mucinous cancer, or can be found alongside another type of breast cancer called invasive breast cancer (no special type). This may be referred to as ‘mixed’ mucinous breast cancer. Pure mucinous breast cancer is less likely to spread to the lymph nodes under the arm than most other types of breast cancer, particularly if the cancer is small, and generally has a better prognosis than most other types of invasive breast cancer.
Other Rarer Subtypes
Metaplastic
A rare variation of IDC which makes up less than less than 5% of all breast cancers. It differs from the more common kinds of breast cancer in both its makeup and in the way it behaves. Like invasive ductal cancer, metaplastic breast cancer begins in the milk duct of the breast before spreading to the tissue around the duct. What makes a metaplastic tumor different is the kinds of cells that make up the tumour. When the cells of an invasive ductal cancer are examined under a microscope, they appear abnormal, but still look like ductal cells. Metaplastic cancers may contain some of these breast cells, too, but they also contain cells that look like the soft tissue and connective tissue in the breast. It is thought that the ductal cells have undergone a change in form (metaplasia) to become completely different cells, though it is not known exactly how or why this occurs.
Metaplastic breast tumours are often, although not always, triple negative. This means that the cancers don’t have receptors for oestrogen, progesterone, or HER2. So hormone therapy or targeted therapy isn’t helpful for these cancers. Metaplastic breast cancer tends not to spread to the lymph glands, but it is more likely to spread to other parts of the body than other types of breast cancer. Metaplastic breast cancers can also behave more aggressively than other kinds of breast cancers, and are, on average, larger at diagnosis.

Adenoid Cystic
Adenoid cystic breast cancer is a rare type of invasive ductal breast cancer which accounts for less than 1% of all breast cancers. It is generally, but not always, seen in older people. When the cells of an adenoid cystic tumour are examined under the microscope, they look like cancer cells more commonly found in the salivary glands. These cells are different than those of typical ductal cancers. Adenoid cystic tumours are often “triple negative”, meaning that the cells do not express the estrogen receptor, progesterone receptor, or HER2 receptor. Even when triple negative, adenoid cystic breast cancers are less likely to involve the lymph nodes, are more responsive to treatment, and may have a better prognosis than more common types of invasive ductal cancer.
Papillary
Papillary breast cancer makes up less than 1% of breast cancers, and tend to affect older women. The term papillary breast cancer can refer to a number of different types of breast cancer.
These include:
- Invasive papillary breast cancer
- Invasive micropapillary breast cancer
- Intracystic/encapsulated/encysted papillary cancer
- Papillary ductal carcinoma in situ
These are often seen alongside other types of breast cancer. The treatment and outlook for papillary breast cancer will depend on the type of papillary breast cancer as well as its features. Papillary breast cancer is not the same as the benign condition intraductal papilloma.
Cribriform
Cribriform breast cancer is a rare type of breast cancer. It’s usually slow growing and low grade, with a good prognosis after treatment. In this type of IDC, the cancer cells invade the connective tissues of the breast as large nests with punched-out holes, which make the tumour look like Swiss cheese. Cribriform breast cancer may be mixed with other types of breast cancer. If it’s not mixed with another type, it’s called pure cribriform. Cribriform cancer cells can also be found in a type of early breast cancer called DCIS (ductal carcinoma in situ).
Apocrine
Apocrine breast cancer is a rare type of invasive ductal breast cancer. Like other types of invasive ductal cancer, apocrine breast cancer begins in the milk duct of the breast before spreading to the tissues around the duct. When the cells of an apocrine tumour are examined under the microscope, they look like cells normally found in the sweat glands in the underarm and groin region. It is thought that the normal ductal breast cells have undergone a change in form, called metaplasia, to become more like apocrine cells, though it is not known exactly how or why this occurs. Apocrine cancers are often “triple negative”, meaning that the cells do not express the oestrogen receptor, progesterone receptor, or HER2 receptor. Apocrine tumour cells are almost always positive for an additional receptor called the “androgen” receptor. Apocrine tumours, even when triple negative, are less likely to involve the lymph nodes, are more responsive to treatment, and may have a better prognosis than more common types of invasive ductal cancer.
Paget’s Disease
Paget’s disease of the nipple is a rare condition which causes skin changes in the nipple area (bleeding, itching, flaking, crusting and nipple discharge) and accounts for fewer than 3% of all breast cancers. While not a cancer itself, Paget’s disease of the breast (Paget’s disease of the nipple) is a rare “carcinoma in situ” in the skin of the nipple, commonly associated with an underlying breast malignancy, either preinvasive (DCIS) or invasive.
Often, Paget’s is mistaken for eczema or an infection before the correct diagnosis is made. Under the microscope, Paget’s cells are often found to be high grade, which means that they look very different from normal cells, and are dividing rapidly. About half are found to be positive for oestrogen and progesterone receptors, and most are positive for the HER2 protein. An important part of the diagnosis and staging of Paget’s disease includes checking for another tumour elsewhere in the breast. Most of the time, there is another cancer, even if one is not seen on mammography, so other tests, including breast MRI are recommended.
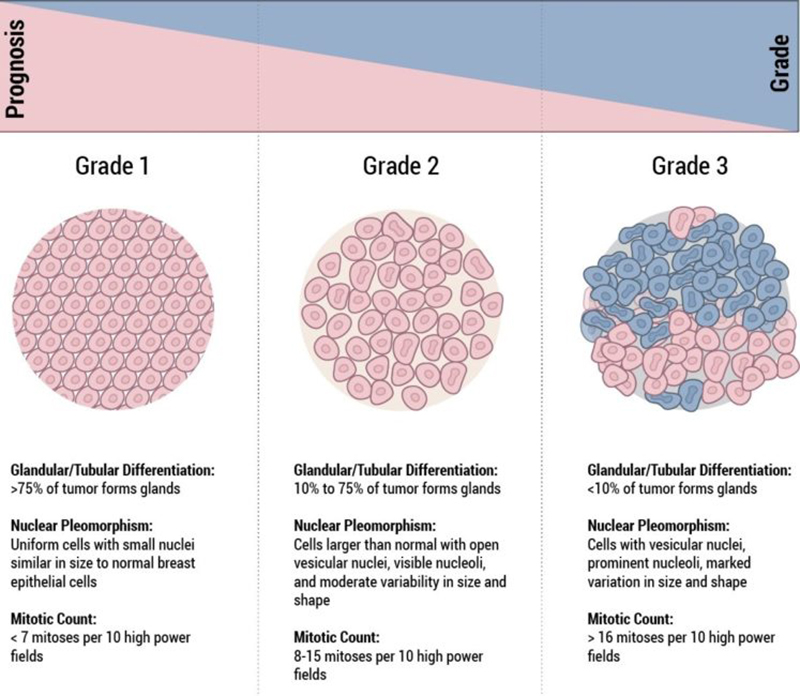
Histological Grade
The grade of a breast cancer is a prognostic factor and is representative of the “aggressive potential” of the tumour. In general “low grade” cancers tend to be less aggressive than “high grade” cancers. When looking at the cancer cells under the microscope, the pathologist looks for certain features that can help predict how likely the cancer is to grow and spread. These features include the arrangement of the cells in relation to each other, whether they form tubules (gland formation), how closely they resemble normal breast cells (nuclear grade), and how many of the cancer cells are in the process of dividing (mitotic count). These features taken together determine how differentiated the cancer is, and its grade.
- Well-differentiated carcinomas have relatively normal-looking cells that do not appear to be growing rapidly and are arranged in small tubules for ductal cancer and cords for lobular cancer. These cancers tend to grow and spread slowly and have a better prognosis (outlook).
- Poorly differentiated carcinomas lack normal features, tend to grow and spread faster, and have a worse prognosis.
- Moderately differentiated carcinomas have features and a prognosis in between these two.
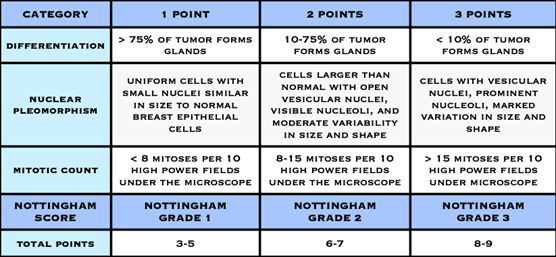
Histological grade is reported using the “Nottingham Score”, also known as the Elston-Ellis modification of the Scarff-Bloom-Richardson grading system, after Chris Elston and Ian Ellis, two pathologists at the Nottingham Breast Unit where Jane O’Brien worked for several years.
It is a combination of nuclear grade, mitotic rate, and tubule formation, which are characteristics of the tumour cells seen under a microscope that reflect/predict its aggressiveness. The scoring system is detailed and you may see it on the report and be interested in what it means. In general, high grade tumours are more likely to recur when compared to low grade tumours.
- Nuclear Grade: a score is given from 1 to 3, based on the appearance of the nucleus of the cancer cells, with 1 being the closest to normal cells (better), 3 being the most variation (worse)
- Mitotic Rate: describes how quickly the cancer cells are multiplying or dividing using a 1 to 3 scale, 1 being the slowest, 3 the most rapid
- Tubule formation: this score represents the percent of cancer cells that are in tubule formation. A score of 1 means greater than 75% of cells are in tubule formation (better), a score of 3 is used when less than 10% of cells are in tubule formation (worse), a score of 2 is in between 10 and 75%
The three scores are then combined for a total score between 3 (1+1+1) and 9 (3+3+3). This score translates to a histological grade. You may see the three values and total score or just the final grade.
- Score of 3,4 or 5: Well differentiated or low grade (Grade 1)
- Score of 6 or 7: Moderately differentiated or intermediate grade (Grade 2)
- Score of 8 or 9: Poorly differentiated or high grade (Grade 3)
Nottingham Grading System
Note: The grade is not the same as the stage of the cancer. The stage indicates whether the cancer is confined to the breast or has moved to the lymph nodes or to another part of the body. The stage cannot be fully assessed until the cancer has been removed and examined under a microscope and the lymph node status is known. Sometimes other investigations such as a CT or a bone scan may be required to complete the staging, but this is not always necessary.
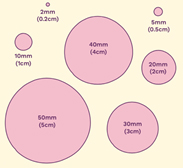
Tumour Size
The size of the cancer is reported in mm and cm.
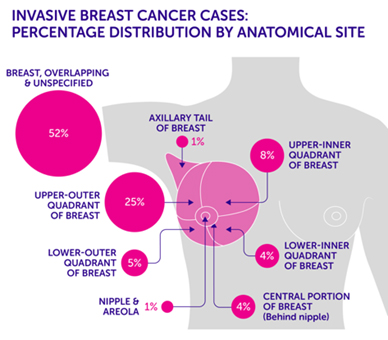
Tumour locations are often given based on the quadrant it was found in. Imagine the breast is divided with a “+” sign into 4 parts or quadrants. They are named upper inner quadrant (UIQ), upper outer quadrant (UOQ), lower outer quadrant (LOQ), lower inner quadrant (LIQ) and “axillary tail” is used to describe the breast tissue that extends towards the armpit.
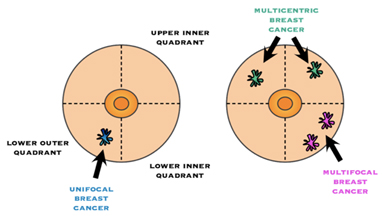
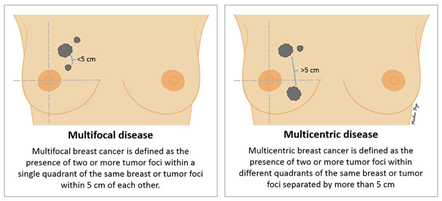
Multifocality / Multicentricity
Even if has not been appreciated preop, it is not uncommon for the pathologist to find additional tumour(s) in the specimen that you did not know were there. If multiple tumours are found, the size and location of each will be noted. “Multifocal” means more than area, but only in one quadrant of the breast or the tumour foci are within 5cm of each other, and “multicentric” means there is more than one area of breast cancer in different quadrants of the breast or when the tumour foci are separated by more than 5 cm.
The American Joint Committee on Cancer TNM guidelines are used to stage all breast cancers, regardless of histology. The assessment of tumour size (T) category may be more complicated in multifocal or multicentric tumours. In such cases, the T category is based on the size of the single largest mass, not an additive sum of multiple tumours.
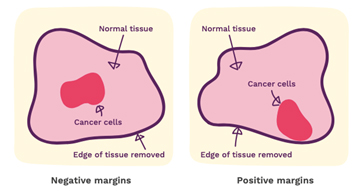
Margins
Your report will give some information about the margins. These are the edges of the surgical specimen and the report will tell you how close the cancer comes to the edge. When performing cancer surgery, the surgeon attempts to remove the entire tumour and some normal tissue surrounding it. This area of “normal tissue” is important because any stray cancer cells may be included in this. If the edge (or margin) contains tumour, there may have been cancer cells left behind. The goal of surgery is to achieve a “clear margin”, that is, clear of any cancer cells. A “clean” or “clear” margin is defined as no tumour cells at the edge of the specimen. If the tumour cells are at the margin, additional surgery may be needed.
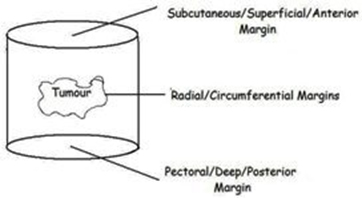
When wide local excision of a breast cancer is performed, a cylinder of breast tissue including the tumour
is removed (see right). There is no further tissue superficially (the excision extends up to just below the skin) or deeply (the excision extends down to chest wall). The superficial and deep pathological margins are therefore not usually relevant as no further tissue can be taken from those margins. It is the radial margins (described in the pathology report as superior, inferior, medial and lateral) that are important, and if any of these margins are involved or inadequate, further surgery is likely to be considered.
Lymphovascular Invasion
When the pathologist examines the tumour and surrounding tissue available to them, they look at the tiny blood vessels and lymphatic drainage to see if any tumour cells have invaded them. This is different from the lymph nodes and would be reported as whether or not lymphatic or vascular invasion is seen. The presence of this may be a sign of a more aggressive tumour.
Lymph Nodes
The lymph system is essentially the “housekeeping system” of the body. It is a network of vessels which connect lymph nodes. These nodes can vary in size, but are normally up to about 2 centimetres in width. They contain cells that clear bacteria and other foreign debris from the body. Lymph is a watery liquid that flows between cells in the body, picking up foreign debris and taking it into the lymph node for filtering and ultimately, elimination by the liver.
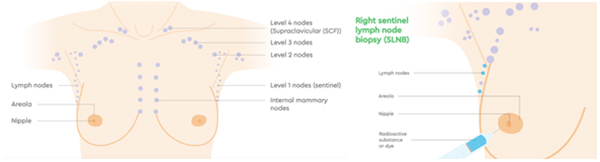
Cancer cells use the lymph system as a first step to travelling to other areas of the body. During breast cancer surgery, lymph nodes are removed and checked for the presence of cancer cells. This will be reported as the number of lymph nodes that contained cancer cells and how many were examined. For example, the report might state “ten benign lymph nodes (0/10)” (no cancer seen) or “tumour seen in ten of twelve lymph nodes (10/12).”
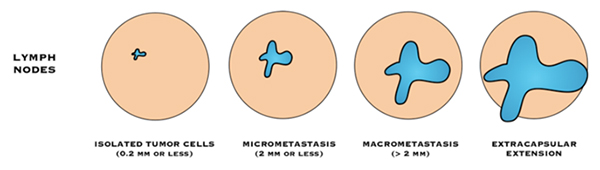
“Negative” lymph nodes means the nodes tested do not contain cancer cells, or contain very few cancer cells (known as isolated tumour cell clusters or ITCs).
- Isolated tumour cells (ITC): means there are fewer than 200 cancer cells are in the lymph node, or the cancer in the lymph node is 0.2 mm or smaller in size.
“Positive” lymph nodes mean there are cancer cells in the nodes.
- Macrometastasis: If there is a breast cancer deposit >2mm, in a lymph node, it is described as a “macrometastasis”
- Micrometastsais: If there are 200 cancer cells or more, in the lymph node, or the cancer in the lymph node is between 0.2 mm and 2 mm in size it is called a “micrometastasis”.
If cancer cells are found in the tissue surrounding the lymph nodes, it is called extra-capsular or extranodal spread.
In most cases, sentinel lymph node biopsy (SLNB) will be used. This procedure involves injecting a radioactive tracer and a blue dye into the breast and allowing it to naturally drain to the lymph nodes. The first lymph nodes it travels to are called the sentinel node(s). The theory is that the cancer cells would travel the same path, so if cancer cells are not present in the sentinel node, it can be safely assumed that they have not spread into the lymph system.
If the pathologist finds cancer cells in the sentinel node, there is a discussion post operatively as to whether further action is required. Axillary lymph node dissection (ALND) can now be safely avoided in many situations. This is largely based on findings from the ACOSOG Z0011 trial, in which patients with clinical T1/2 N0 disease undergoing breast conserving surgery followed by radiation, with up to 2 positive sentinel nodes, fared equally well with SLNB alone or ALND, with low rates of both locoregional recurrence and nodal recurrence, irrespective of treatment.
For patients with positive sentinel nodes not fitting the ACOSOG Z0011 criteria, the AMAROS trial aimed to determine whether axillary radiotherapy (RT) can replace ALND for patients with positive nodes on SLNB. The study found low axillary recurrence rates in both arms (<2%); however, the risk of lymphoedema with ALND was nearly double that of axillary radiation (23% vs 11% ).
Tumour Characteristics
Hormone Receptor Status
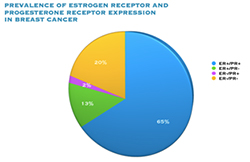
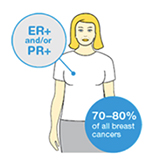
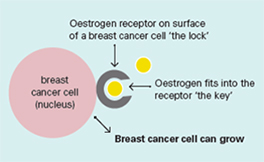
Oestrogen and progesterone are hormones that drive the growth of most breast cancers, stimulating cancer cells to grow by binding their specific receptors, the oestrogen receptor (ER) and the progesterone receptor (PR). Hormone receptors for oestrogen and progesterone are present in high numbers in some breast cancers, making the growth of these tumours reliant on hormones. These tumours are referred to as hormone receptor positive (HR+) and can be ER+/PR+, ER+/PR- or rarely ER-/PR+.
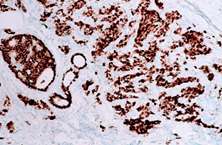
ER+ cancer (immunostain)
The receptors are present on the cancer cells and when the hormone attaches to the receptor, it allows the cancer cell to grow and divide. Hormone therapy can be used to interfere with these receptors, slowing or stopping tumour growth, and preventing recurrence, and the ER and PR expression predicts that the patient will likely benefit from endocrine therapy that targets the hormone receptor, such as tamoxifen and others. Approximately 70-80% of breast cancers express ER and/or PR, and these cancers have a more favourable prognosis than if the tumour is “ER and PR negative.” ER+/PR+ breast cancer is associated with a more favourable prognosis than ER+/PR- breast cancer, and is associated with significantly improved outcomes as compared to ER-/PR- breast cancer.
Oestrogen receptors on the surface of the nucleus inside the cell allow oestrogen from the bloodstream to fit into these receptors like a key fits into a lock. This causes the breast cancer cells to grow.
There is no universal standard for reporting the receptor status, so you may see any of the following:
- A percentage of the cells that reacted positive for receptors (from 0% to 100%)
- A number between 0 and 3, with 0 being no receptors and 3 being the most receptors. An Allred score is a combination of the percent positive and their intensity. The score is from 0-9, with 9 being the most strongly receptor positive
- Positive or negative: In the case of just a positive or negative result, the percentage should be requested. This is because research has shown that even tumours with very low positivity can benefit from hormone therapy, yet some labs report low results (<10%) as negative.
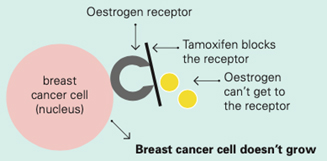
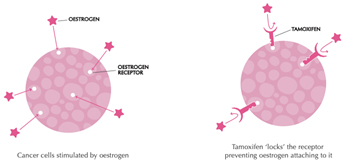
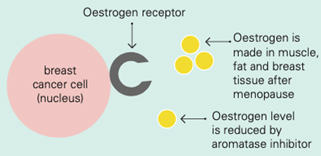
Tamoxifen works by blocking the oestrogen receptor so that oestrogen can’t make the cancer cell grow
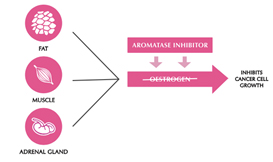
Aromatase inhibitors and treatments to remove or turn off the ovaries work by lowering the level of oestrogen in the blood. This means that oestrogen is not available to fit into the receptor to make the cancer cell grow
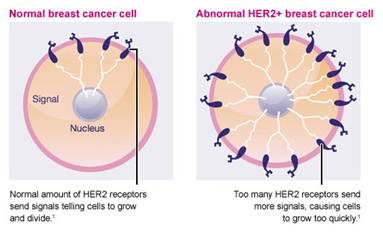
HER2 Status
Guide to Understanding HER2-Positive Breast Cancer
Onco Link HER2-Positive Breast Cancer
The HER2 gene stimulates production of a protein found on the surface of breast cancer cells that tells the cells to grow and divide. In about 15-20% of breast cancers, there are too many copies of the gene and the protein is over expressed on the cell surface, causing the cancer to grow faster, and be more aggressive. HER-2 overexpression is seen in approximately 15-20% of invasive breast cancers.
Breast tumours are routinely tested to see if they have too many copies of the gene or over express the protein.
The immunohistochemistry (IHC) test looks for overexpression of the protein, and is reported as a number from 0 to +3.
- 0 or 1+ is considered HER2 negative
- 2+ is equivocal/borderline
- 3+ is considered HER2 positive
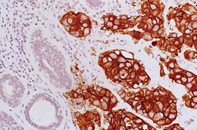
HER2+ cancer (immunostain)
HER2+ cancer-fluorescence in situ hybridization (FISH)
Breast cancers with a 2+ or 3+ result on IHC are retested using more specialised tests, called ISH (situ hybridisation) which examine the tumour for extra copies of the HER2 gene.
ISH tests are reported as either:
- positive
- negative
These are regarded as the definitive tests, on which the final decision regarding treatment will be made. HER2 positive tumours are treated with medications, including monoclonal antibodies, targeting the HER2 protein eg Herceptin.
Triple Negative Breast Cancer
Some breast cancers are hormone receptor negative (ER– and PR–) and HER2 negative (HER2–). These are called triple negative breast cancers (TNBC).
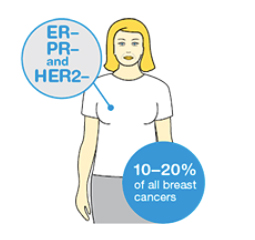
Triple negative cancers do not respond to hormone therapy or to targeted therapy drugs used for HER2 positive cancers. The current treatment options for people with triple negative breast cancer include chemotherapy before or after surgery and some other types of targeted therapy drugs.
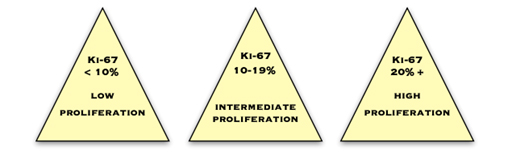
Ki67
Ki67 is a protein which is expressed in cells which are proliferating or about to proliferate. The pathologist estimates the percentage of cancer cells which contain this protein. The more positive cells there are, and the higher the score, the more quickly the cancer is dividing and growing. Commonly, less than 10% is considered low, 10–20% is medium and more than 20% is high.
Molecular / Intrinsic Breast Cancer Subtypes
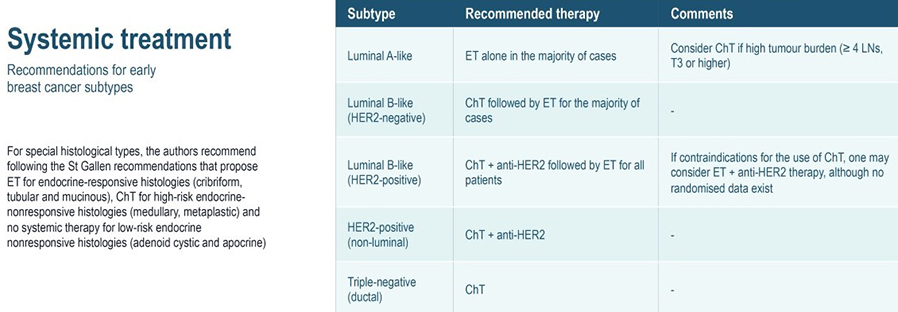
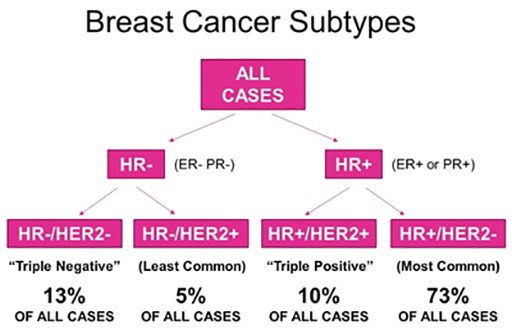
In clinical practice, early-stage breast cancers are divided into three or four basic subgroups based on expression of oestrogen receptor (ER), progesterone receptor (PR), and HER2. Tumours are classified as ER and/or PR positive and HER2 negative, HER2-positive, or by default, triple-negative breast cancer (TNBC). Approximately half of HER2 positive tumours are also ER positive. These categorisations have definitive consequences for systemic treatment.
- Nearly all ER positive tumours will be candidates for adjuvant endocrine therapy.
- The majority of TNBCs will warrant adjuvant chemotherapy, and the majority of HER2 positive cancers warrant anti-HER2 therapy in combination with chemotherapy.
In a landmark article in Nature in 2000, Perou and colleagues published the first article classifying breast cancer into intrinsic subtypes based on gene expression profiling using DNA microarrays. The newer classification model captures tumour biology in a more comprehensive way than the expression of a single protein or gene alone. The molecular subtype of an invasive breast cancer is based on the genes the cancer cells express, which control how the cells behave.
Gene expression profiling has reshaped our understanding of breast cancer by identifying four molecular subtypes:
- Luminal A (50%)
- Luminal B (20%)
- Human Epidermal growth factor Receptor 2 (HER2)-enriched (15%)
- Basal-like (15%)
The four molecular subtypes have critical differences in incidence, response to treatment, disease progression and survival. Subtypes can be defined by genetic array testing or approximations to this classification using immunohistochemistry. These subtypes have different epidemiological risk factors, different natural histories, and different responses to systemic and local therapies. These differences imply that clinicians managing breast cancer should consider cases within the various distinct subtypes in order to properly assess the relevant evidence and arrive at appropriate therapeutic advice.
Studies show that the three main variables predictive of outcome in early breast cancer include tumour size, nodal status, and now intrinsic subtype. Characterization of breast cancer into four intrinsic molecular subtypes began a new era in breast cancer research and created a paradigm shift in the clinical approach to treatment.
- Luminal tumours are the most common (60%–70%), characterized by oestrogen receptor (ER) expression. Luminal A tumours have the best prognosis of all subtypes, whereas patients with luminal B tumours have significantly shorter overall and disease-free survival. Distinguishing between these tumours is important, because luminal B tumours require more aggressive treatment.
- HER2 enriched tumours are characterized by overexpression of the HER2 oncogene. HER2+ disease carries a poorer prognosis, but the development of anti-HER2 therapies has greatly improved outcomes for women with HER2+ breast cancer.
- Basal-like cancers (15% of all invasive breast cancers) predominate among “triple negative” cancers, which lack ER, progesterone receptor (PR), and HER2 expression. Basal-like cancers are frequently high-grade, with high rates of recurrence.
The Luminal Subtypes
Genetic Expression
The distinguishing characteristic of the luminal subtype is ER expression. The majority of luminal tumours demonstrate genetic expression of ER and progesterone receptor (PR). The luminal subtype is the most common subtype of breast cancer and is divided into at least two distinct subgroups, A and B. Luminal A tumours are more common, comprising roughly 40-50% of all breast cancers, compared to 20% for luminal B tumours. Luminal A and B breast cancers have important differences in gene expression patterns and clinical prognosis. Luminal A-type tumours are deemed to have a favourable prognosis, and they are typically low-grade invasive ductal carcinomas (not otherwise specified type) or special types, including tubular, cribriform and mucinous, and also classical lobular carcinomas. This subtype is responsive to endocrine therapy but has a poor response to traditional chemotherapy.
Genetic expression of hormone receptors (HR) is a shared feature of luminal A and B tumours. However, luminal B tumours are distinct from the luminal A subtype by having a higher expression of proliferative and/or cell-cycle genes and a lower expression of PR, and typically higher grade invasive ductal NST or micropapillary carcinomas.
Clinical Implications and Management
Multiple studies have shown that the subtypes of ER+ tumours, luminal A and B, have two distinct clinical courses. Patients with luminal B tumours have significantly shorter overall survival (OS) and disease-free survival (DFS) times compared to patients with luminal A breast cancer. Of all breast cancer subtypes, luminal A tumours have the best prognosis, whereas luminal B, HER2 enriched, and basal subtypes are associated with poorer clinical outcomes. The overexpression of cell cycle and proliferation genes in luminal B tumours is thought to account for the poorer prognosis of patients with luminal B breast cancer and the more aggressive nature of these tumours compared to luminal A. Given the distinctly different prognoses of the luminal subtypes, it is clinically important to distinguish patients with luminal A tumours from those with luminal B.
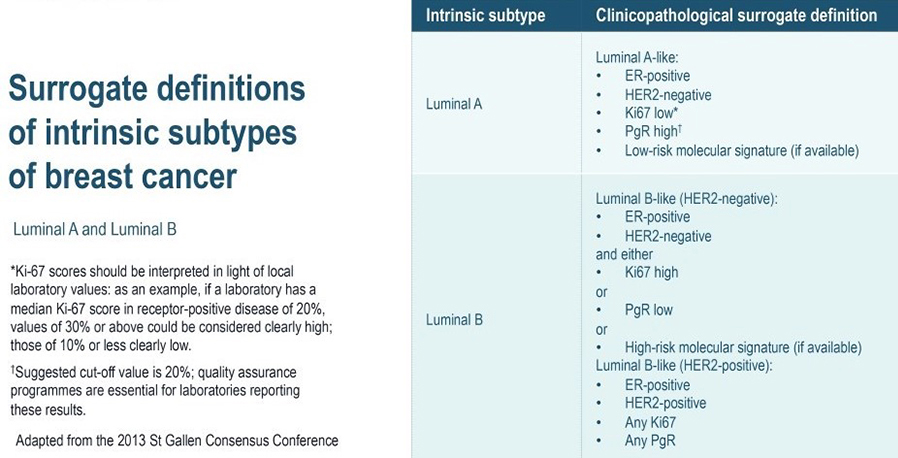
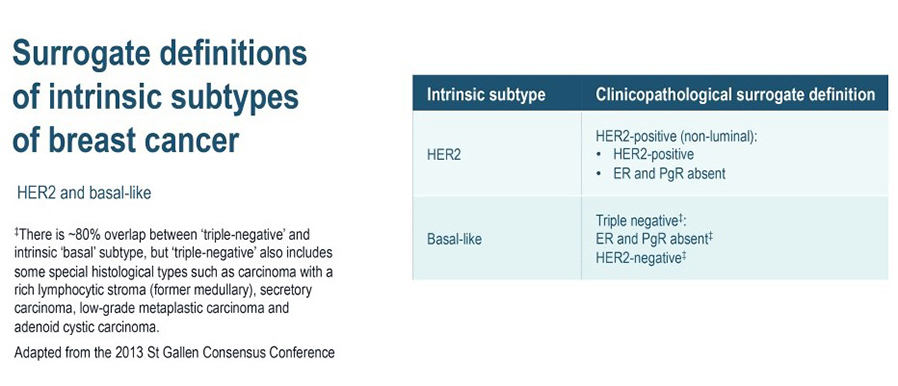
The four intrinsic/molecular subtypes are defined by gene expression profiling. Because gene expression profiling is not available to most practicing physicians, the molecular classification has been adapted to clinical practice on the basis of frequently used biomarker assays: ER, PR, HER2, and some measure of proliferation, usually the Ki-67 assay. A simplified classification has thus been adopted as a useful shorthand using immunohistochemistry. Subtypes defined by clinicopathological criteria are similar to, but not identical to intrinsic subtypes, and represent a convenient approximation.
As summarized in the table above, semiquantitative immunohistochemical (IHC) expression of ER, PR, HER2, and Ki-67 are used to define surrogates of the four molecular subtypes of breast cancer. Differences depend upon the choice of the threshold value for Ki-67 and the requirement for substantial PR positivity, and the initial surrogate classification has undergone subsequent revisions to make it more relevant.
In clinical practice, luminal B tumours are distinguished from luminal A tumours by a higher histologic grade, lower PR expression (20% or less), and higher Ki-67 expression (typically >20%) However, IHC surrogates do not always accurately reflect the true intrinsic molecular subtype. Discordance rates between IHC analysis and gene expression profiles can be as high as 30%.
Given the useful but imperfect nature of IHC surrogates, gene expression profiles are sometimes needed to guide clinical decisions. The most common scenario in which a gene expression profiles is ordered is an ER+, PR+, HER2-, lymph-node-negative patient with an intermediate Ki-67 (10%–30%). Gene assays such as Oncotype Dx and Endopredict, provide prognostic information and risk assessment. Oncotype Dx is used to inform the risk of recurrence by providing a score, which guides whether a patient can be treated with hormone therapy only (low recurrence score) or would benefit from the addition of chemotherapy (high recurrence score).
The AJCC Cancer Staging Manual, 8th edition recognized that incorporating biomarkers into the TNM classification system was necessary. This manual proposed a new prognostic stage to describe breast cancer that combines histologic grade, ER status, PR status, HER2 status, and multigene assays (specifically Oncotype Dx) with anatomic TNM staging .
As a clinical ‘short-hand’, tumours are often classified as ‘luminal- A like’ or ‘luminal-B like’ based on routine pathology. Luminal A-like tumours are typically low grade, strongly ER/PR positive, HER2 negative with a low proliferative fraction. Luminal-B-like tumours are ER positive but may have variable degrees of ER/PR expression, are higher grade, and have a higher proliferative fraction. These classifications based on routine histopathology are clinically valuable, but caution needs to be exercised, particularly for example when considering the option of neoadjuvant chemotherapy in the case of a potentially luminal B cancer, when the pathological information available on the core biopsy may be limited.
Low PR in isolation does not determine a tumour to be definitively luminal B, and newer clinicopathological definitions to distinguish the luminal A and luminal B intrinsic molecular breast cancer subtypes have been developed, most using 20% as a threshold for Ki-67 to distinguish the luminal A and luminal B intrinsic molecular breast cancer subtypes, with a requirement for substantial PR positivity in the definition of luminal A like disease, only when Ki-67 expression is intermediate, between 14% and 20%. Others in addition include histological grade.
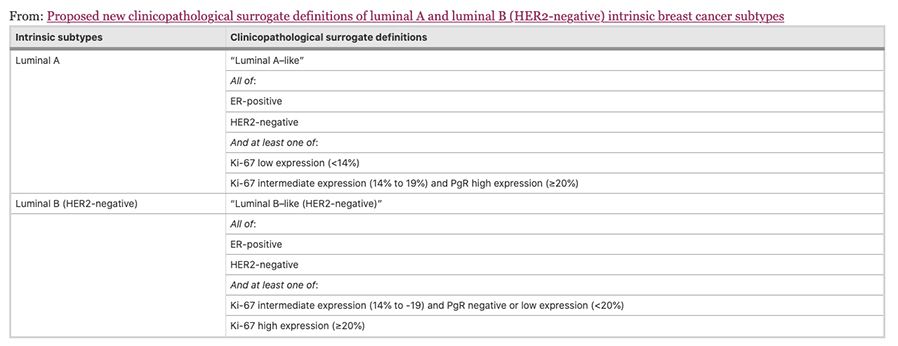
Maisonneuve et al, Breast Cancer Research 2014
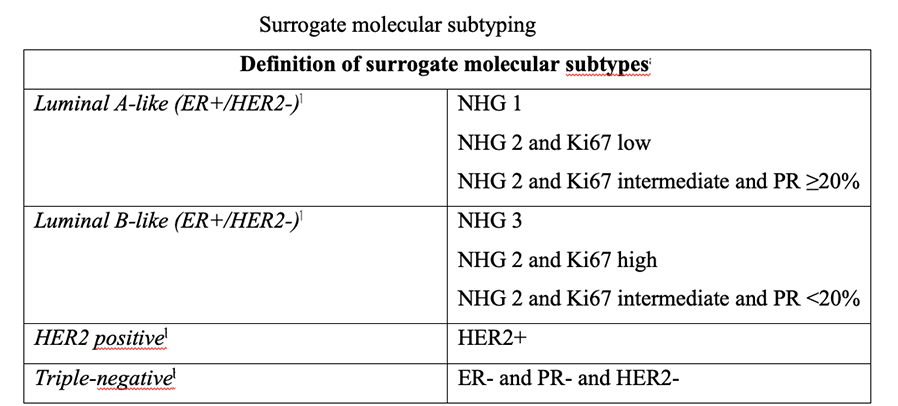
NHG (Nottingham Histological Grade)
Since 2011, the St. Gallen international expert consensus panel has adopted an intrinsic subtype-based approach for recommending adjuvant systemic therapies (i.e. endocrine, chemotherapy and anti-HER2 therapy) in early breast cancer. Although the the superior accuracy and reproducibility of multi-gene expression molecular assays is acknowledged, these assays are not readily available for all our patients. Thus, over the years, pathology-based surrogate definitions especially for distinguishing Luminal A from B tumours have been proposed. However, despite important efforts to improve the various pathology-based surrogate definitions of the intrinsic subtypes, these continue to be suboptimal.
Studies show that the three main variables predictive of outcome in early breast cancer include tumour size, nodal status, and now intrinsic subtype. Recognizing the four intrinsic molecular subtypes of breast cancer—luminal A, luminal B, HER2-enriched, and basal-like—has begun to unravel the heterogeneity of breast cancer and will lead to further development of targeted treatments, improving the prognosis of all women diagnosed with breast cancer.
Breast Cancer Staging
Breast cancer staging describes how far the cancer has spread within the breast and other parts of the body. It is an important factor in making treatment decisions. Breast cancer staging is based on tumour size, the extent that cancer has spread to other parts of the body and other clinical factors. Breast cancer staging can be pathologic or clinical.
Types of Staging
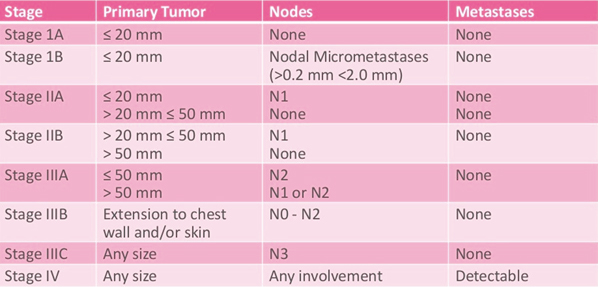
There are 2 main types of staging systems for cancer. These are the TNM system and the Number system.
The systems mean that:
- doctors have a common language to describe the size and spread of cancers
- doctors can compare treatment results between research studies
The TNM staging system
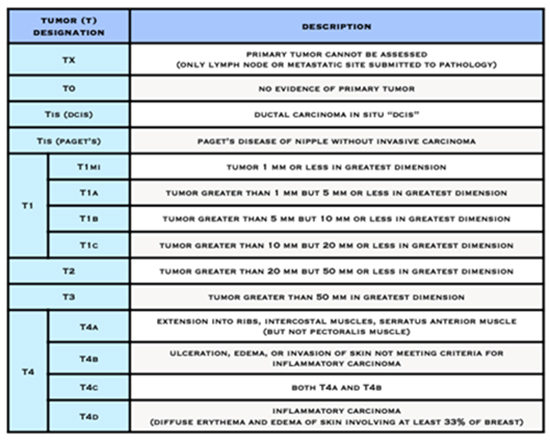
The breast cancer staging system, called the TNM system, is overseen by the American Joint Committee on Cancer (AJCC). The AJCC is a group of cancer experts who oversee how cancer is classified and communicated. This is to ensure that all doctors and treatment facilities are describing cancer in a uniform way so that the treatment results of all people can be compared and understood.
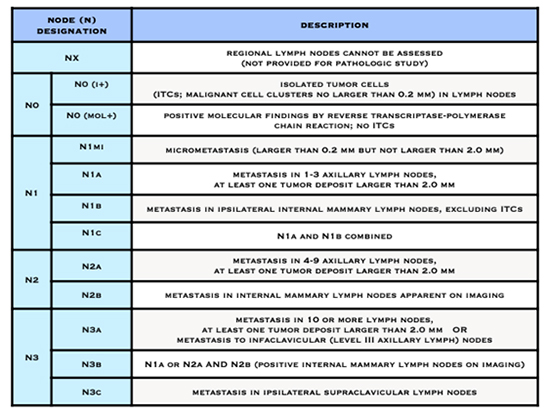
The T, N, and M letters in the staging system stand for:
- the size of the cancer tumour and whether it is invasive (T) or non-invasive (Tis)
- whether cancer is in the lymph nodes (N)
- whether the cancer has spread to other parts of the body beyond the breast (metastasized) (M)
Numbers or letters after T, N, and M give more details about each characteristic. Higher numbers mean the cancer is more advanced.
- The letter T followed by a number from 0 to 4 describes the tumour’s size, and spread to the skin or to the chest wall under the breast. Higher T numbers mean a larger tumour and/or wider spread to tissues near the breast.
- The letter N followed by a number from 0 to 3 indicates whether the cancer has spread to lymph nodes near the breast and, if so, how many lymph nodes are affected.
Clinical stage
Clinical staging is based on the results of any tests done before surgery, such as an exam by your doctor or mammogram or MRI results. Clinical stage is determined by your doctor and is labelled with the prefix “c” before TNM. Clinical staging information is not included in the pathology report.
Pathologic Stage
The pathologic stage of a breast cancer is determined by the pathologist and is labelled with the prefix “p.” The pTNM stage is determined by the cancer’s characteristics, most importantly by how large it is and whether cancer cells are in the lymph nodes. The purpose of the staging system is to help guide treatment decisions and provide a common way to describe breast cancer so treatment results can be compared. Your doctor uses the pathologic stage information to determine a clinical stage to help make treatment decisions.
The information contained in your breast cancer operative pathology report, together with the result of scans such as CT chest, abdomen and pelvis and bone scan or PET/CT scan, which check for the presence of tumour elsewhere in the body, allows for your breast cancer to be “staged”.
If you have neoadjuvant treatment before surgery to remove the breast cancer, then the stage is determined after treatment and is reported as ypTNM. The “y” stands for “after treatment.”
American Joint Committee on Cancer (AJCC) Staging System for Breast Cancer
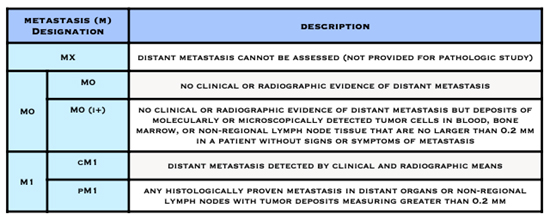
The Number Staging System
The number staging system uses the TNM system to divide breast cancers into 4 stages.

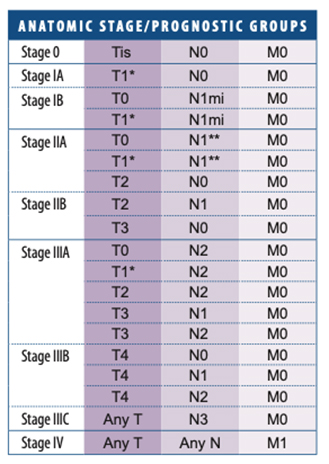
In recent years, breast carcinoma pathology reports from open breast biopsy, ‘breast conservation’ and mastectomy specimens have been increasingly standardised and expressed in summary or ‘synoptic’ format. Following the pathologist’s description of the macroscopic appearance of the specimen, there is usually a summary of the critical features used to make treatment decisions. Each report should therefore contain all the important prognostic and predictive information required to guide prognosis and treatment.
Special Circumstances
The Surgical Operative Pathology Report after Neoadjuvant Therapy: Understanding Your Pathology Report After Neoadjuvant Therapy
Sometimes drug therapy in the form of chemotherapy, HER2-targeted therapy, immunotherapy and/or hormone therapy, is given before surgery, for example to shrink a larger breast cancer and/or tumour in the axillary lymph nodes.
After surgery, the tissue is checked. Your pathology report will tell you the size of any cancer still present. On the report this is sometimes called the “residual” size of the cancer.
The pathology report after neoadjuvant therapy includes:
- Tumour bed size: The overall size of any remaining cancer found during surgery after neoadjuvant treatment.
- Tumour bed cellularity: The percentage of cancer cells found in the tumour bed.
How much breast cancer remains will indicate the response to the treatment you had before surgery. This is called the pathological response:
- Complete pathological response means no remaining invasive This is referred to as a “pathological complete response” (pCR)
- Partial response means only some of the cancer remains
- No evidence of response means the cancer is the same or bigger than before neoadjuvant therapy
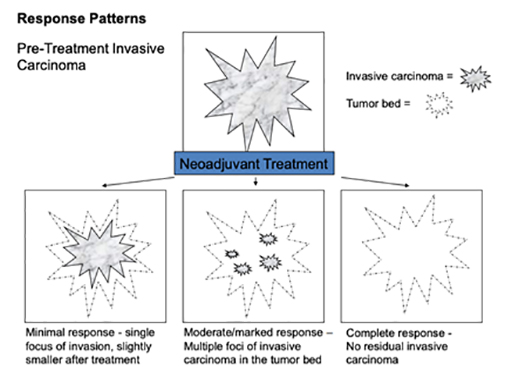
Tissue that has experienced a partial response to neoadjuvant therapy continues to have invasive carcinoma present, however the extent of tumour has decreased, the cellularity of the tumour has diminished, and reactive fibrosis or sclerosis may be present. Tissue that does not demonstrate a pathologic response to neoadjuvant treatment appears very similar to the original biopsy specimen, without substantial change in tumour size and cellularity, and without local inflammation or fibrotic change.
Breast cancers treated with neoadjuvant therapy are staged a bit differently than those treated with surgery first. Before neoadjuvant therapy begins, the pre-treatment stage of your breast cancer may be determined using imaging (such as mammograms) and findings from a physical exam of the breast. This is called clinical prognostic stage.
After surgery, the stage of your breast cancer is determined using pathology information from the tissue removed during surgery. The pathologic stage after neoadjuvant therapy gives the most information on your prognosis.
TNM ( Tumour size, lymph Node status and Metastases) Staging System and Neoadjuvant Therapy
When TNM is used after neoadjuvant therapy, you’ll see a “y” before the T and N measures on your pathology report. Otherwise, the categories are the same as those for tumours not treated with neoadjuvant therapy.
yT = Tumour stage after neoadjuvant therapy
yN = Lymph node status after neoadjuvant therapy
Metastatic status is determined before treatment begins.
M0 = No metastases
M1 = Metastases
Tumour size after surgery
A pathologist will study the tissue removed during breast cancer surgery and take measurements. Some samples of the tissue may need to be studied under a microscope to check for small amounts of cancer. The largest length of the tumour is the tumour size.
On your pathology report, the post-neoadjuvant treatment tumour size will be indicated with “ypT” followed by numbers and sometimes, letters. The “y” notes the measurement was taken after neoadjuvant therapy. The “p” means a pathologist took the measurement from the tissue removed during surgery.
Sometimes, there’s more than one area of cancer in the breast. The largest continuous area will be reported as the tumour size. You’ll see the letter “m” to note there were multiple areas of cancer in the breast.
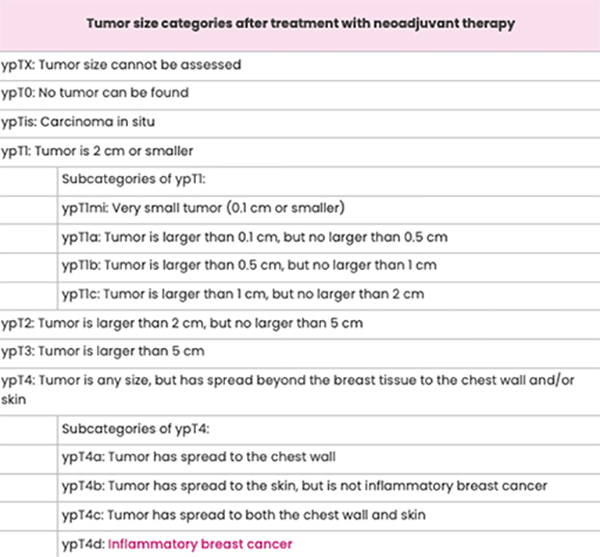
Lymph Node Status after Surgery
The definition for each category of lymph node status is the same whether or not you get neoadjuvant therapy. The only difference is the “yp” notation before the “N” on your pathology report. For example, pN0 and ypN0 both mean the axillary and other nearby lymph nodes don’t contain cancer.
The pre-treatment status of the lymph nodes may be included on your pathology report. This is called clinical node status and is determined using imaging (for example, with breast ultrasound or breast MRI) and findings from a physical exam. Clinical node status is noted as “cN” on a pathology report.
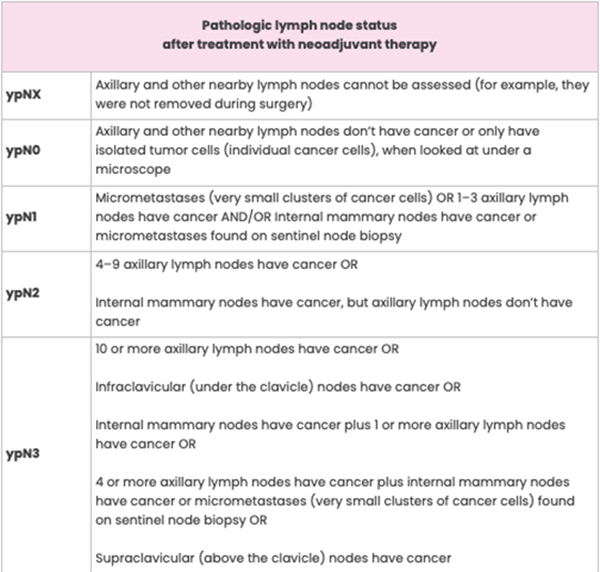
Pathologic Complete Response to Neoadjuvant Therapy
Sometimes, the pathologist’s exam shows no sign of cancer in the breast or axillary lymph nodes. This is called a pathologic complete response (pCR) and means the neoadjuvant therapy eradicated all of the breast cancer. If you have a pCR, it will be noted on your pathology report.
Although a pCR is encouraging, it doesn’t mean the cancer will never return, and many people who don’t have a pCR will still do very well.
pCR rates after neoadjuvant chemotherapy are highest among women with tumours that are:
- High-grade
- Hormone receptor-negative (oestrogen receptor-negative and/or progesterone receptor-negative)
- HER2-positive, when the neoadjuvant treatment plan includes trastuzumab and pertuzumab
The standardized definition of pathological complete response (pCR) includes residual preinvasive disease/DCIS (either ypT0/is ypN0 or ypT0ypN0)
The most widely accepted definition of pathologic complete response (pCR) includes absence of residual invasive disease in the breast and the sampled axillary nodes (ypT0/is, ypN0), but includes residual preinvasive disease/DCIS. Patients who achieve a pCR have lower rates of systemic and local recurrence, and pCR predicts for excellent survival, regardless of subtype.
Residual Breast Cancer after Neoadjuvant Therapy
Any breast cancer that remains in the breast or axillary lymph nodes after neoadjuvant therapy is called residual breast cancer. Many people have some residual breast cancer after neoadjuvant therapy. Your pathology report will include whether or not residual breast cancer was found in the breast and/or lymph nodes.
Residual Cancer Burden
Sometimes, information about residual cancer burden (RCB) is included on your pathology report. An RCB score is determined using information on the size of the tumour and the extent of tumour cells in the breast and axillary lymph nodes after neoadjuvant therapy.
The higher the RCB score, the more residual breast cancer there is in the breast and lymph nodes:
- RCB-0 = No residual breast cancer
- RCB-I = Small amount of residual breast cancer- partial response
- RCB-II = Moderate amount of residual breast cancer- partial response
- RCB-III = Extensive residual breast cancer – chemoresistant
The Residual Cancer Burden is an online tool for the quantification of residual disease that is simple to apply, reproducible, and has been clinically validated with long-term follow-up data. The RCB system requires the use of a formula. A web-based calculation script is available to calculate the scores ( http://www.mdanderson.org/breastcancer_RCB).
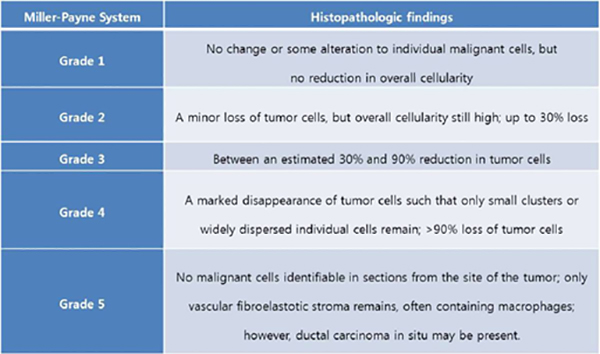
Miller-Payne Score
The Miller-Payne Score divides the reduction in overall cellularity into five grades, with grade 1 as no significant reduction in overall cellularity, and grade 5 as a complete pathologic response at the site of the primary tumour. This system does not include the response in lymph nodes.
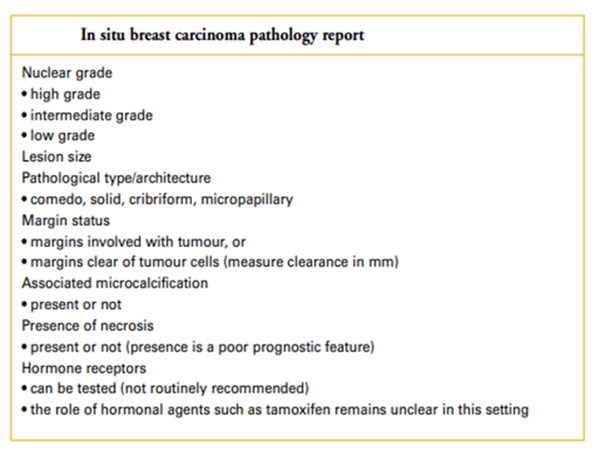
The “in situ” and “invasive” breast carcinoma pathology reports should include all the information found in the tables below

BCNA Breast Cancer Pathology 2019
Putting it all together
By understanding the basics of your pathology report, you will be better able to discuss your treatment options with your healthcare team. Remember that all the information in the pathology report is considered together when deciding about which treatments to offer you and their likely benefits. No one piece of information should be looked at on its own in isolation – it always needs to be related to all the other information.
- Breast Cancer Pathology
- Understanding Your Pathology Results
- A Guide to your Breast Cancer Pathology Report
- What does my pathology report mean?
- Understanding Your Pathology Report: Breast Cancer
- Glossary Of Pathology Terms
You will need the Adobe Reader to view and print these documents.
![]()